Fig. 3.
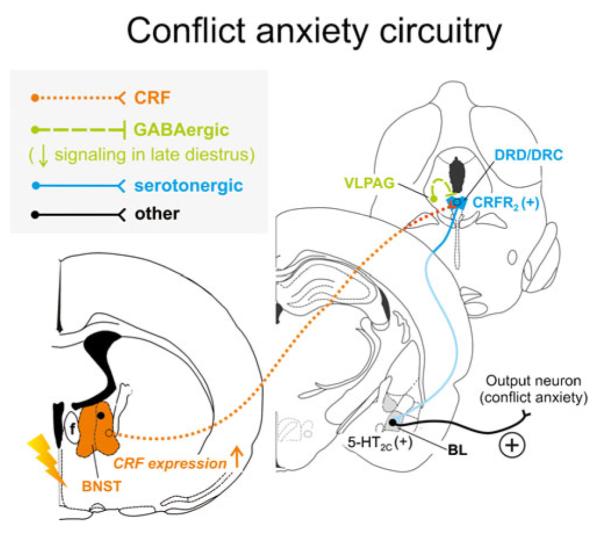
Hypothetical model of how stress-induced increases in corticotropin-releasing factor (CRF) expression and signaling from the bed nucleus of the stria terminalis (BNST) may interact with decreased GABAergic inhibition from the ventrolateral periaqueductal gray (VLPAG) during late diestrus to enhance serotonergic output in the conflict anxiety-related dorsal and caudal DR (DRD/DRC). During late diestrus in rodents, declining circulating concentrations of progesterone and its neuroactive metabolite allopregnanolone cause increased expression of α4, β1, and δ subunits of the γ-aminobutyric acid (GABA) receptor type A within the PAG [143, 144], including the VLPAG, ultimately resulting in attenuated ongoing GABAergic inhibitory signaling [221]. Attenuated activity of GABAergic neurons from the VLPAG render serotonergic neurons in the DRD/DRC more active [183], and stress induces CRF expression in the conflict anxiety-related BNST [232, 331, 375]. Enhanced CRF release from BNST projections further activates serotonergic neurons through CRF receptor type 2 (CRFR2) [204] within the DRD/DRC [335]. Together with stress-induced desensitization of autoinhibitory 5-HT1A receptors on DRD/ DRC serotonin neurons [313], late diestrus-enhanced hyperactivity of the DRD/DRC causes increased serotonergic output to distal target sites controlling conflict anxiety-like behavior, in particular through actions on excitatory 5-HT2C receptors [76] in the BL [12, 150]. Neuronal projections are drawn unilaterally solely for simplicity and do not imply functional laterality
