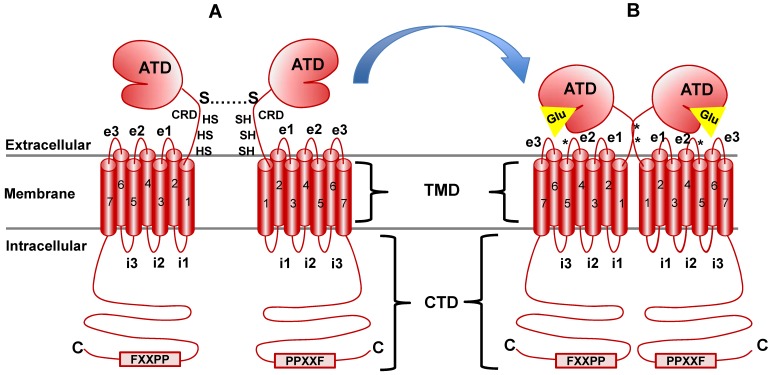
Fig 4.
mGluR structure and conformational activation. mGluR family members share the same basic structure: Beginning at the N terminus (N), the amino terminal domain (ATD) is followed by the cysteine rich domain (CRD), the seven transmembrane domains (TMD; numbered) and the C-terminal domain (CTD). i1, i2, etc., indicate intracellular loops between membrane-spanning regions while e1, e2, etc., indicate extracellular loops. The star at loop e2 denotes the cysteine residue that is important in transmitting activation information to the docked G protein complex (see text for detail). Panel A illustrates the native resting conformation of mGluR1a. Cysteine residues in the CRD aid in dimerization, as indicated by the S-S bond. Upon Glu binding (Panel B), a conformational change in the ATD involving the CRD (stars indicate disulfide bridges created by Glu binding) causes activation of bound G proteins (see text for details).