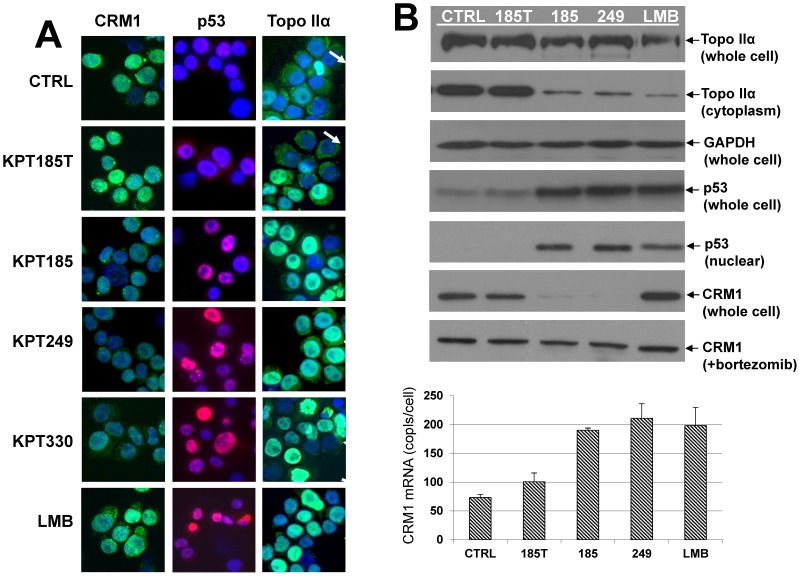
Fig 2.
CRM1, p53, and topo IIα localization in SINE-treated cells. A, Immunofluorescence microscopy. Human H929 MM cells were treated with SINE or leptomycin B for 20 hours. Samples assayed for p53 and CRM1 were treated with 100 nM SINE and 10 nM LMB. Samples assayed for topoisomerase (topo) IIα were treated with 300 nM SINE and 100 nM LMB. Intracellular localization and expression of p53, CRM1, and topo IIα were examined by immunofluorescence microscopy. Nuclei were counter-stained using DAPI (blue). Column 1: CRM1 (green) nuclear localization in low-density log-phase cells (2 x 105/mL) was increased in untreated controls, in KPT185T-treated cells, and in LMB-treated cells, but not in KPT185-, KPT249-, or KPT330-treated cells. Column 2: p53 (red) was exported from the nucleus in low-density log-phase (2 x 105/mL) untreated control, and KPT185T-treated cells; however, cells treated with KPT185, KPT249, KPT330, and LMB had increased nuclear accumulation of p53. Column 3: high-density (3 x 106/mL) untreated control cells exported topo IIα (green) to the cytoplasm (as did KPT185T treated cells) and had low levels of topo IIα in the nuclei (arrows) (blue/DAPI). KPT185, KPT249, KPT330, and LMB prevented nuclear export of topo IIα; therefore, the nuclei were green (topo IIα) (arrows). B, CRM1, p53, and topo IIα protein expression in SINE-treated cells. H929 cells were treated with 100 nM of each CRM1 inhibitor for 20 hours at high-density (3 x 106/mL) growth conditions. Whole cell lysates assayed for topo IIα protein demonstrated minimal change in total amount of topo IIα; however, cytoplasmic fractions showed that the nuclear export of topo IIα was inhibited by KPT185, KPT249, and LMB. GAPDH protein (loading control) showed that equal amounts of protein were loaded for topo IIα. H929 cells were also treated with 100 nM of each CRM1 inhibitor for 20 hours at log-phase growth conditions. Whole cell lysates assayed for p53 showed that total cellular p53 increased in cells treated with CRM1 inhibitors when compared to untreated and KPT185T-treated control samples. Nuclear fractions isolated from treated cells and assayed by Western blot demonstrated that nuclear p53 increased when cells were exposed to the active CRM1 inhibitors KPT185, KPT249, and LMB. KPT185 and KPT249, but not LMB or KPT185T, decreased CRM1 protein expression in whole cell lysates. However, when cells were treated with the proteasome inhibitor bortezomib, CRM1 protein levels did not decrease. Treated cells were also assayed for CRM1 mRNA levels. We found that all CRM1 inhibitors had significantly (P < 0.05) increased mRNA levels as compared to untreated and KPT185T-treated controls.