Abstract
It has been suggested that whole-genome duplication (WGD) occurred twice during the evolutionary process of vertebrates around 450 and 500 million years ago, which contributed to an increase in the genomic and phenotypic complexities of vertebrates. However, little is still known about the evolutionary process of homoeologous chromosomes after WGD because many duplicate genes have been lost. Therefore, Xenopus laevis (2n=36) and Xenopus (Silurana) tropicalis (2n=20) are good animal models for studying the process of genomic and chromosomal reorganization after WGD because X. laevis is an allotetraploid species that resulted from WGD after the interspecific hybridization of diploid species closely related to X. tropicalis. We constructed a comparative cytogenetic map of X. laevis using 60 complimentary DNA clones that covered the entire chromosomal regions of 10 pairs of X. tropicalis chromosomes. We consequently identified all nine homoeologous chromosome groups of X. laevis. Hybridization signals on two pairs of X. laevis homoeologous chromosomes were detected for 50 of 60 (83%) genes, and the genetic linkage is highly conserved between X. tropicalis and X. laevis chromosomes except for one fusion and one inversion and also between X. laevis homoeologous chromosomes except for two inversions. These results indicate that the loss of duplicated genes and inter- and/or intrachromosomal rearrangements occurred much less frequently in this lineage, suggesting that these events were not essential for diploidization of the allotetraploid genome in X. laevis after WGD.
Keywords: Xenopus laevis, Xenopus tropicalis, comparative gene mapping, FISH, whole-genome duplication, homoeologous chromosomes
Introduction
Whole-genome duplication (WGD: polyploidization) is an evolutionary event that played an important role in the diversification of most eukaryotic lineages (Ohno, 1970; Kellis et al., 2004; Kasahara, 2007; Otto, 2007). It is generally accepted that each duplicated gene evolved independently after WGD and that the polyploid genome quickly turned into a diploid state, referred to as diploidization, through repeated genomic and chromosomal reorganization including the loss of homoeologs, with some genes being consistently maintained as duplicates. Diploidization through genomic and chromosomal reorganization after ancient WGDs has been identified in yeast and most plant genomes (Song et al., 1995; Seoighe and Wolfe, 1998; Pontes et al., 2004; Simillion et al., 2004; Yu et al., 2005; Fischer et al., 2006; Tuskan et al., 2006; Woodhouse et al., 2010). A total of 80% of the duplicated genes in yeast were lost after WGD 80 million years ago (Mya) (Kellis et al., 2004), 70% in Arabidopsis after WGD 86 Mya (Bowers et al., 2003) and 47% in maize after WGD 5–12 Mya (Woodhouse et al., 2010).
Polyploidization is less prevalent in animals than in plants. Comparative analyses of genome sequences in vertebrates and chordates revealed that WGD occurred twice (2R-WGD) in the early evolutionary history of vertebrates around 450 and 500 Mya, and 3R-WGD occurred in the teleost fish lineage >350 Mya after 2R-WGD (Ohno, 1970; International Human Genome Sequencing Consortium, 2001; Jaillon et al., 2004; Kasahara et al., 2007; Putnam et al., 2008). Comparative genome analyses of four teleost fish species (medaka fish, Takifugu, Tetraodon and zebrafish) suggested that genomic and chromosomal reorganization frequently occurred in the teleost fish lineage after 3R-WGD (Kasahara et al., 2007; Sémon and Wolfe, 2007). It has been suggested that the common ancestor of salmonid fishes underwent one additional WGD 50–100 Mya after 3R-WGD (Allendorf and Thorgaard, 1984). Comparisons of genetic linkage between salmonid fishes (Atlantic salmon and rainbow trout) and other teleost fishes (medaka, stickleback and zebrafish) revealed that the genetic linkage of teleost fishes is conserved in salmonid fishes, although several chromosomal rearrangements occurred after WGD in salmonid genomes (Danzmann et al., 2008; Guyomard et al., 2012). However, little is known about the process of genomic and chromosomal reorganization after WGD in vertebrates because many duplicate genes derived from WGD have been lost in vertebrate genomes. For example, genome sequence analyses revealed that 76–80% of duplicated genes derived from 3R-WGD have been lost in extant teleost fish lineages, and it is speculated that approximately 60% of these duplicated genes were rapidly lost within about 75 million years after 3R-WGD (Jaillon et al., 2004; Sato et al., 2009).
The African clawed frog (Xenopus laevis, Pipidae, Anura) (2n=36) and the western clawed frog (Xenopus (Silurana) tropicalis, Pipidae, Anura) (2n=20) are widely used as experimental animals in a wide range of scientific fields such as developmental, cellular, immunological and molecular biological research. More than 20 species of extant clawed frogs classified into two genera (Silurana and Xenopus) have been reported, which exhibit a variable number of chromosomes (2n=20–108). It is suggested that these species except for X. tropicalis resulted from polyploidization, which occurred after the hybridization of two different species (Tymowska and Fischberg, 1973; Bisbee et al., 1977; Tymowska, 1991; Hughes and Hughes, 1993; Kobel, 1996; Evans et al., 2004, 2008). The allotetraploidization events occurred at least twice in clawed frogs after the divergence of the ancestor of the diploid species X. tropicalis, which has 20 chromosomes and the ancestor of diploid species now thought to be extinct, which had 18 chromosomes (Tymowska, 1991; Evans, 2008), and this divergence probably occurred 50–65 Mya (Evans et al., 2004; Hellsten et al., 2007). In Silurana, X. tropicalis and another diploid species underwent allopolyploidization to give rise to three tetraploid species with 40 chromosomes, including X. epitropicalis and two undescribed species. Allopolyploidization between two 18-chromosome species occurred at least once 21–40 Mya, giving rise to the ancestor of all Xenopus species with 36, 72 or 108 chromosomes (Evans et al., 2004; Chain and Evans, 2006; Hellsten et al., 2007). Therefore, X. laevis and X. tropicalis are good animal models for understanding the process of genomic and chromosomal reorganization after WGD. We recently constructed a high-resolution chromosome map consisting of 140 genes for X. tropicalis (Uno et al., 2012). The draft genome assemblies of X. tropicalis were reported previously (Hellsten et al., 2010), and several comparative studies of complimentary DNA (cDNA) sequences have been performed between X. tropicalis and X. laevis (Morin et al., 2006; Hellsten et al., 2007; Sémon and Wolfe, 2008). However, the information is still insufficient to know genetic linkage of X. laevis, and no comprehensive comparative analyses using genomic sequencing or cytogenetic mapping have been conducted for X. laevis and X. tropicalis.
In this study, we constructed a comparative cytogenetic map of X. laevis by fluorescence in situ hybridization (FISH) mapping of the functional genes that had been localized to X. tropicalis chromosomes. We consequently identified all nine homoeologous chromosome groups of X. laevis and then revealed the chromosome rearrangements that occurred between the two species and also between the homoeologous chromosomes of X. laevis. We discussed the process of genomic and chromosomal evolution in the X. laevis lineage after WGD on the basis of our comparative cytogenetic map of X. laevis.
Materials and methods
Animals, cell culture and chromosome preparation
We used adult females of the J strain of X. laevis, which were purchased from the breeder. After pithing, heart, lung and kidney tissues were collected for cell culture. All experimental procedures using animals conformed to the guidelines established by the Animal Care Committee, Nagoya University. Tissues were minced, and cells were cultured at 26 °C in a humidified atmosphere of 5% CO2 in air for 10–14 days (Uno et al., 2008). Primary cultured cells were harvested using 0.5% trypsin and then subcultured. For replication-banded chromosome preparation, 5-bromo-2′-deoxyuridine (25 μg ml−1) was added to the cell cultures at log phase, and cell culturing was continued for 6 h including 1 h colcemid treatment (0.17 μg ml−1) before harvesting. Chromosome preparations were made following a standard air-drying method. After staining with Hoechst 33258 (1 μg ml−1) for 5 min, slides were heated to 65 °C for 3 min on a hot plate and then exposed to ultraviolet light for an additional 5–6 min at 65 °C (Matsuda and Chapman, 1995). Slides were kept at −80 °C until use.
Fluorescence in situ hybridization
For chromosome mapping, 60 X. laevis cDNA clones were selected from 140 clones that were used for the chromosome mapping of X. tropicalis in our previous study (Uno et al., 2012). These clones were isolated based on a web data catalog of the NIBB/NIG/NBRP Xenopus laevis EST project (XDB3, http://xenopus.nibb.ac.jp/). FISH mapping was performed as described previously (Matsuda and Chapman, 1995). DNA probes were labeled with biotin-16-dUTP (Roche Diagnostics, Basel, Switzerland) using a nick translation kit (Roche Diagnostics) following the manufacturer's instruction and ethanol precipitated with sonicated salmon sperm DNA and Escherichia coli transfer RNA. After hybridization, the hybridized probes were reacted with a goat anti-biotin antibody (Vector Laboratories, Burlingame, CA, USA) and then stained with Alexa Fluor 488 rabbit anti-goat IgG (H+L) conjugate (Molecular Probes, Life Technologies, Carlsbad, CA, USA). Chromosome slides were counterstained with 0.75 μg ml−1 propidium iodide. To discriminate the Z and W chromosomes, a 15-kb genomic DNA fragment of the DM-W gene (Yoshimoto et al., 2008) was hybridized to the chromosome slide, where the hybridization signal was detected on chromosome 3. The hybridized probe was removed from the slides by redenaturation in 70% formamide/4 × standard saline citrate at 70 °C for 2 min, and then the DM-W probe was hybridized with a 100-time volume of the sonicated whole genomic DNA of X. laevis to the same slide. After hybridization, slides were incubated with fluorescein isothiocyanate–avidin (Roche Diagnostics), and hybridization signals were observed.
Results
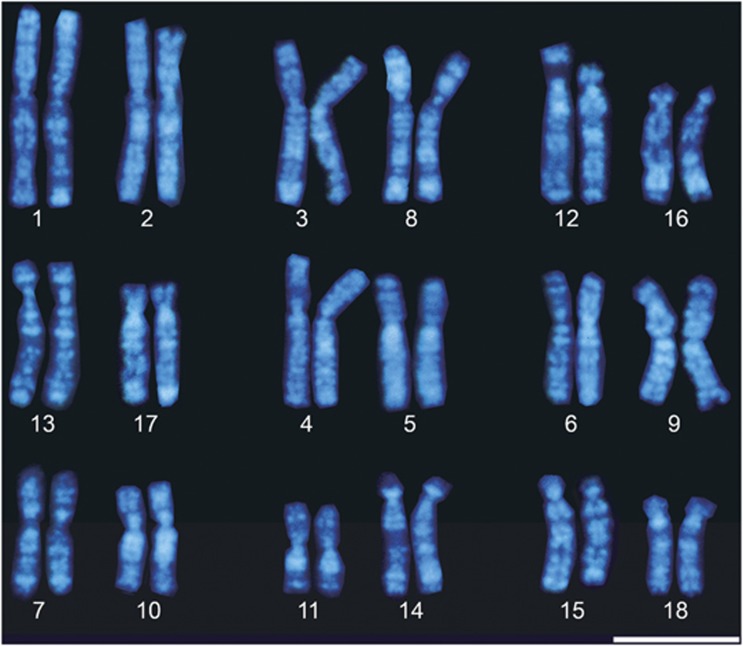
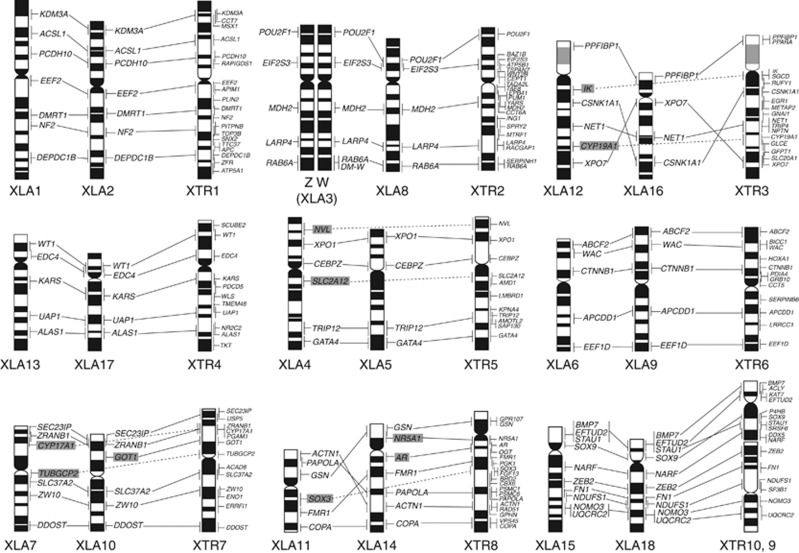
High-resolution Hoechst-stained bands of X. laevis chromosomes were obtained using a replication banding method (Figure 1), and their ideograms were slightly modified from our previous one (Uno et al., 2008). Based on our previous mapping data of 140 genes in X. tropicalis (Uno et al., 2012), we selected 60 genes, which covered the entire chromosomal regions of 10 pairs of X. tropicalis chromosomes, for their comparative mapping to X. laevis chromosomes (XLA) (Figure 2 and Table 1). The identification of each chromosome and subchromosomal localization of the hybridization signals was performed using the Hoechst G-banded ideogram. Hybridization signals were detected on two pairs of homoeologous chromosomes for 83% of the cDNA clones (50/60) (Figure 3). For instance, seven genes (KDM3A, ACSL1, PCDH10, EEF2, DMRT1, NF2 and DEPDC1B), which were mapped on X. tropicalis chromosome (XTR) 1, were all localized to XLA1 and XLA2, indicating that XLA1 and XLA2 are homoeologous. All the other homoeologous chromosome pairs of X. laevis and their homologies with X. tropicalis chromosomes were as follows: XTR2 was homologous to XLA3 and XLA8, XTR3 to XLA12 and XLA16, XTR4 to XLA13 and XLA17, XTR5 to XLA4 and XLA5, XTR6 to XLA6 and XLA9, XTR7 to XLA7 and XLA10 and XTR8 to XLA11 and XLA14. The genes on XTR9 and XTR10 were all localized to XLA15 and XLA18, suggesting that the homoeologous chromosome pair XLA15 and XLA18 was derived from a tandem fusion of XTR9 and XTR10 or that XTR9 and XTR10 are derivatives of a fission event that occurred in an original chromosome pair of homoeologous XLA15 and XLA18.
Figure 1.
Hoechst-stained replication-banded karyotype of the female X. laevis (XLA). Each chromosome was numbered following our previous karyotypic study of X. laevis (Uno et al., 2008). Chromosomes were grouped as homoeologous chromosome pairs (XLA1+2, 3+8, 12+16, 13+17, 4+5, 6+9, 7+10, 11+14 and 15+18) according to comparative gene mapping in this study (Figure 3). Small size variation of chromosomes between individuals was found for XLA12p (Uno et al., 2008), which depends on the size differences of the 18S–28S ribosomal gene cluster on the short arm (data not shown). Scale bar=10 μm.
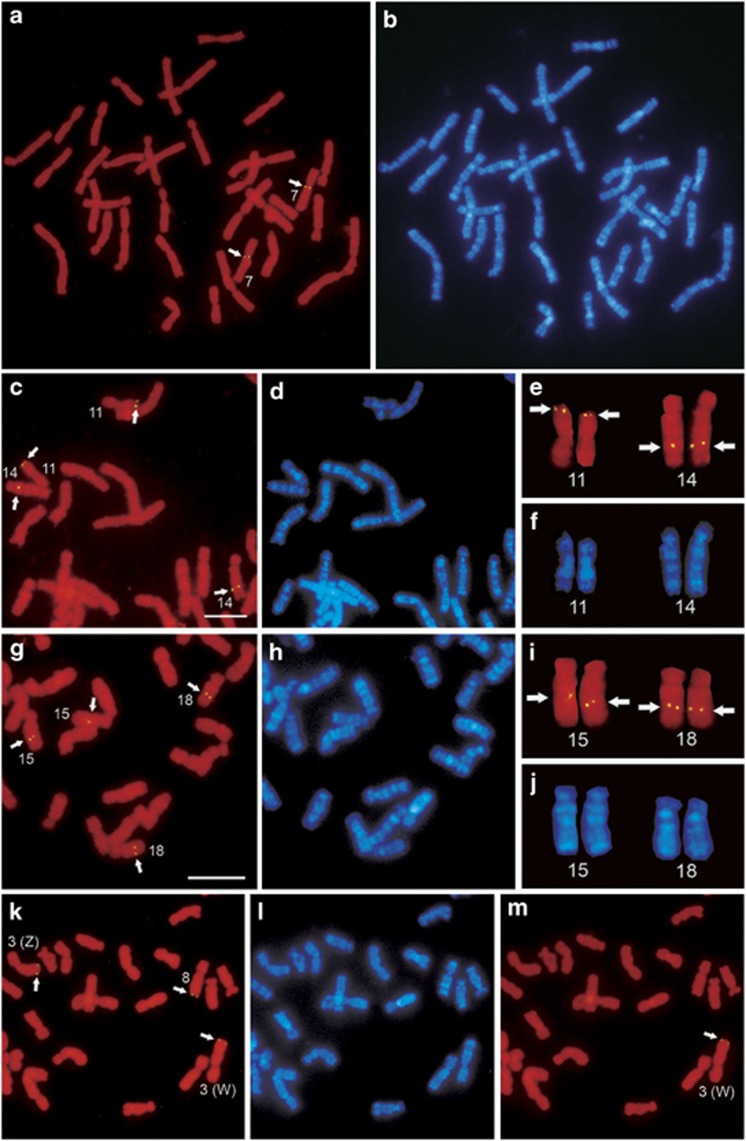
Figure 2.
Chromosomal localization of DNA clones to metaphase chromosome spreads of the female X. laevis. FISH patterns of the cDNA clones of TUBGCP2 on chromosome 7p (a), ACTN1 on chromosomes 11p and 14q (c, e), FN1 on chromosomes 15q and 18q (g, i) and RAB6A on chromosomes 3q and 8q (k) on propidium iodide-stained metaphase chromosome spreads. Arrows indicate hybridization signals. The FISH pattern of a genomic DNA fragment of the DM-W gene on chromosomes 3q (m) on the same metaphase spread that was used for mapping of the RAB6A gene (k). The DM-W probe was hybridized to the metaphase spread after the RAB6A probe was removed. The Z and W sex chromosomes were precisely identified by FISH with the DM-W probe. Hoechst-stained patterns of the propidium iodide-stained chromosomes in panels a, c, e, g, i and k are shown in panels b, d, f, h, j and l, respectively. Scale bars=10 μm.
Table 1. List of 60 genes localized to X. laevis chromosomes.
| Gene symbola | Clone no.b or accession no. |
Chromosomal location |
|||
|---|---|---|---|---|---|
| X. laevis | X. tropicalisc | Chickend | Humand | ||
| KDM3A | XL413k20ex | 1p, 2p | 1p | 4q | 2p11.2 |
| ACSL1 | XL318g05ex | 1p, 2p | 1p | 4q | 4q34–q35 |
| PCDH10 | XL003c24 | 1p, 2p | 1p | 4q | 4q28.3 |
| EEF2 | XL470l07ex | 1q, 2q | 1q | 28 | 19pter–q12 |
| DMRT1e | AB201112 | 1q, 2q | 1q | Zq | 9p24.3 |
| NF2 | XL085b04 | 1q, 2q | 1q | 15 | 22q12.2 |
| DEPDC1B | XL220a24 | 1q, 2q | 1q | Zp | 5q12.1 |
| POU2F1 | XL164e23 | 3p, 8p | 2p | 1q | 1q22–q23 |
| EIF2S3 | XL408e08ex | 3p, 8p | 2p | 1q | Xp22.2–p22.1 |
| MDH2 | XL165g11 | 3q, 8q | 2q | 19 | 7cen–q22 |
| LARP4 | XL014a12 | 3q, 8q | 2q | un | 12q13.12 |
| RAB6A | XL038d18 | 3q, 8q | 2q | 1q | 11q13.3 |
| PPFIBP1 | XL062p09 | 12p, 16p | 3p | 1p | 12p11.23–p11.22 |
| IK | XL168o22 | 12q | 3q | 13 | 5q31.3 |
| CSNK1A1 | XL221g09ex | 12q, 16q | 3q | 13 | 5q32 |
| NET1 | XL227m13ex | 12q, 16q | 3q | 1p | 10p15 |
| CYP19A1e | BC079750 | 12q | 3q | 10 | 15q21.1 |
| XPO7 | XL214l11 | 12q, 16q | 3q | 22 | 8p21 |
| WT1e | D82051 | 13p, 17p | 4p | 5p | 11p13 |
| EDC4 | XL473f03ex | 13p, 17p | 4p | 11 | 16q22.1 |
| KARS | XL480m02ex | 13q, 17q | 4q | 11 | 16q23–q24 |
| UAP1 | XL086p04 | 13q, 17q | 4q | 8p | 1q23.3 |
| ALAS1 | XL051o04 | 13q, 17q | 4q | 12 | 3p21.1 |
| NVL | XL479c13ex | 4p | 5p | 3q | 1q41–q42.2 |
| XPO1 | XL294p07ex | 4p, 5p | 5p | 3p | 2p16 |
| CEBPZ | XL039l04 | 4p, 5p | 5p | 3q | 2p22.2 |
| SLC2A12 | XL036o21 | 4q | 5q | 3q | 6q23.2 |
| TRIP12 | XL204l01 | 4q, 5q | 5q | 9 | 2q36.3 |
| GATA4 | XL039m17 | 4q, 5q | 5q | 3q | 8p23.1–p22 |
| ABCF2 | XL012l18 | 6p, 9p | 6p | 2p | 7q36 |
| WAC | XL075d07 | 6p, 9p | 6p | 2p | 10p11.2 |
| CTNNB1 | XL480g03ex | 6p, 9p | 6p | 2p | 3p21 |
| APCDD1 | XL055h12 | 6q, 9q | 6q | 2q | 18p11.22 |
| EEF1D | XL013c21 | 6q, 9q | 6q | 2q | 8q24.3 |
| SEC23IP | XL151i10 | 7p, 10p | 7p | 6 | 10q25–q26 |
| ZRANB1 | XL027a17 | 7p, 10p | 7p | 6 | 10q26.13 |
| CYP17A1e | AF325435 | 7p | 7p | 6 | 10q24 |
| GOT1 | XL151j11 | 10p | 7p | 6 | 10q24 |
| TUBGCP2 | XL008b12 | 7p | 7p | 6 | 10q26.3 |
| SLC37A2 | XL082p15 | 7q, 10q | 7q | 24 | 11q24.2 |
| ZW10 | XL046l12 | 7q, 10q | 7q | 24 | 11q23.2 |
| DDOST | XL005p17 | 7q, 10q | 7q | 21 | 1p36.1 |
| GSN | XL300b05ex | 11p, 14p | 8p | 17 | 9q33 |
| NR5A1e | AB273177 | 14p | 8q | 17 | 9q33 |
| ARe | U67129 | 14q | 8q | 4p | Xq11.2–q12 |
| FMR1 | XL022n18 | 11q, 14q | 8q | 4p | Xq27.3 |
| SOX3e | —f | 11q | 8q | 4p | Xq27.1 |
| PAPOLA | XL035j18 | 11p, 14q | 8q | 5q | 14q32.31 |
| ACTN1 | XL286g19ex | 11p, 14q | 8q | 5q | 14q22–q24 |
| COPA | XL263c24ex | 11q, 14q | 8q | 25 | 1q23–q25 |
| STAU1 | XL175g01 | 15p, 18p | 10q | 20 | 20q13.1 |
| BMP7 | XL056l08 | 15p, 18p | 10p | 20 | 20q13 |
| EFTUD2 | XL300a14ex | 15p, 18p | 10p | 27 | 17q21.31 |
| SOX9e | AB439583 | 15p, 18p | 10q | 18 | 17q24.3–q25.1 |
| NARF | XL210h06 | 15q, 18q | 10q | 18 | 17q25.3 |
| ZEB2 | XL207j23 | 15q, 18q | 9q | 7q | 2q22 |
| FN1 | XL338p06ex | 15q, 18q | 9q | 7p | 2q34 |
| NDUFS1 | XL034k12 | 15q, 18q | 9q | 7q | 2q33–q34 |
| NOMO3 | XL271k19ex | 15q, 18q | 9p | 14 | 16p13 |
| UQCRC2 | XL016d01 | 15q, 18q | 9p | 14 | 16p12 |
Abbreviations: EST, expressed sequence tag; un, unknown chromosomal location.
Human gene symbol.
Clone numbers of X. laevis EST clones used for mapping, which were selected from a web data catalog of the NIBB/NIG/NBRP Xenopus laevis EST project (XDB3, http://xenopus.nibb.ac.jp/) based on the X. tropicalis chromosome map described by Uno et al. (2012). Fragment sizes of all X. laevis EST clones were >1.5 kb.
Chromosomal locations for X. tropicalis taken from previous studies (Uno et al., 2008, 2012).
Chromosomal locations of chicken and human homologs searched with the BLATN program of the Ensembl (http://www.ensembl.org/index.html) and/or the blastn program of NCBI (http://www.ncbi.nlm.nih.gov/) (searched in September 2012).
Genes mapped in our previous studies (Uno et al., 2008; Yoshimoto et al., 2008).
The cDNA fragment of SOX3 was isolated by Koyano et al. (1997).
Figure 3.
Comparative cytogenetic map of X. laevis (XLA) and X. tropicalis (XTR). The chromosomal locations of AR, CYP17A1, CYP19A1, DMRT1, DM-W, NR5A1, SOX3, SOX9 and WT1 in X. laevis and the cytogenetic map of X. tropicalis were taken from our previous studies (Uno et al., 2008; Yoshimoto et al., 2008). XTR9 is inverted to facilitate a comparison of the gene order with those of XLA15 and XLA18. Gene symbols enclosed in gray boxes indicate the genes that were located on only one pair of homoeologous chromosomes. The small size variations of XLA12p and XTR3p between individuals are represented as gray-colored bands in the ideogram (Figure 1; Uno et al., 2008).
Genetic linkage has been highly conserved between the two species and also between homoeologous chromosome pairs of X. laevis. No interchromosomal rearrangements (reciprocal translocations) were detected, and gene orders were identical between homologous chromosomes of X. laevis and X. tropicalis and between X. laevis homoeologous chromosome pairs, except for intrachromosomal rearrangements (inversions) that were detected between XLA12 and XLA16, XLA11 and XLA14, and XTR10 and homoeologous XLA15 and XLA18 pairs. These results indicate that the genetic linkage of X. tropicalis chromosomes has been retained almost intact in X. laevis chromosomes with no interchromosomal translocations after WGD.
Five XTR2-linked genes (POU2F1, EIF2S3, MDH2, LARP4 and RAB6A) were all localized to the homologous chromosomes of XLA3 and XLA8. XLA3 was identified as the Z and W sex chromosomes in our previous study (Yoshimoto et al., 2008); however, no morphological differences have been found between the Z and W sex chromosomes (Schmid and Steinlein, 1991; Uno et al., 2008). We identified the W chromosome by FISH mapping of the W-linked sex (ovary)-determining gene, DM-W (Yoshimoto et al., 2008) (Figure 2m). None of the five genes on the Z or W chromosome differed in chromosomal locations and hybridization efficiencies (data not shown), indicating that structural differentiation hardly occurred between the Z and W chromosomes except for the W-specific region containing the DM-W gene.
Discussion
In this study, we identified all nine quartets (the homoeologous chromosome groups) of X. laevis (XLA1+2, 3+8, 12+16, 13+17, 4+5, 6+9, 7+10, 11+14 and 15+18), whose genetic linkage has been highly conserved in X. tropicalis. Paleontological studies have suggested that X. tropicalis is a more ancient species than other extant polyploid Xenopus species, providing the possibility that the ancestral diploid Xenopus species had 2n=20 chromosomes similar to X. tropicalis (Estes, 1975). Therefore, the chromosome number of X. laevis (2n=36) may have occurred as a result of allotetraploidization of the interspecific hybrid between two different species with 2n=18 chromosomes, which was derived from the fusion of two chromosome pairs of the ancestral diploid species with 2n=20 (Schmid and Steinlein, 1991; Tymowska, 1991). Our results suggest that the ancestral bi-armed chromosome pair of homoeologous XLA15 and XLA18 may have been derived from the fusion of XTR9 and XTR10 in the ancestral species of X. tropicalis. The nine quartets identified in this study were not consistent with those determined by 5-bromo-2′-deoxyuridine/dT replication banding (Schmid and Steinlein, 1991) and cross-species chromosome hybridization with X. tropicalis-derived chromosome painting probes (Krylov et al., 2010). Krylov et al. (2010) demonstrated that XLA11+14 and XLA15+18 were painted with XTR8 and XTR9 probes, respectively, and XLA14 and XLA18 were hybridized with XTR10 paint; however, XTR10 showed homology with XLA15 and XLA18 by comparative gene mapping in the present study. This discrepancy may be due to a difference in the numbering system of X. laevis chromosomes.
High rate of loss of duplicated genes (50–75%) after WGD has been reported in X. laevis using a large number of expressed sequence tags (ESTs) (20 223 ESTs reported by Hellsten et al. (2007) and 28 463 ESTs by Sémon and Wolfe (2008)), which is not so different from the rate of gene loss in teleost fishes after 3R-WGD (76–80%) (Jaillon et al., 2004; Sémon and Wolfe, 2007), yeast after WGD (80%) (Kellis et al., 2004), Arabidopsis (70%) (Bowers et al., 2003) and maize (47%) (Woodhouse et al., 2010). However, in this study, hybridization signals were detected on both homoeologous chromosomes for most clones (50 of 60 genes: 83%), which indicated that the loss of duplicated genes after WGD was much lower in X. laevis (17%). These results suggest that the loss of one copy of duplicated genes could not be determined accurately using only a partial collection of X. laevis gene ESTs (Hellsten et al., 2007). Analyses using both genome sequences and chromosome mapping of a large number of transcribed genes are needed to more accurately estimate the frequency of gene loss in X. laevis after WGD.
Our comparative maps of functional genes between X. tropicalis and X. laevis demonstrated no evidence of interchromosomal rearrangements between two species and revealed that genetic linkage has been highly conserved between X. tropicalis and X. laevis except for inversions between XTR10 and homoeologous XLA15 and XLA18 pairs. The gene orders on X. tropicalis chromosomes have also been highly conserved in two homoeologous pairs of X. laevis chromosomes except for inversions between XLA12 and XLA16 and between XLA11 and XLA14. According to the gene orders of XTR3 and XTR8, which are considered to be the ancestral types of homoeologous XLA12 and XLA16 pairs and XLA11 and XLA14 pairs, respectively, a large paracentric inversion may have occurred in the long arm of XLA16, with at least two inversions containing a pericentric inversion in XLA11. The gene orders of SOX9 and STAU1 and their locations in XTR10q are different from those in XLA15p and XLA18p, suggesting that a pericentric inversion event occurred in the proximal region of the ancestral chromosome of homoeologous XLA15 and XLA18 pairs after the fusion between XTR9 and XTR10 of the ancestral diploid species such as X. tropicalis. These results provide us with the following two possibilities: (1) gene orders were different in some chromosomes between the ancestral diploid species before WGD occurred; and (2) intrachromosomal rearrangements occurred in one of the homoeologous chromosome pairs after WGD. In either case, the present results indicate that inter- and/or intrachromosomal rearrangements occurred much less in the X. laevis lineage for 21–40 million years after allotetraploidization than in teleost fishes (medaka fish, Takifugu, Tetraodon and zebrafish) (Kasahara et al., 2007; Sémon and Wolfe, 2007), salmonid fishes (Danzmann et al., 2008; Guyomard et al., 2012), plants (Arabidopsis, rice and Populus) (Pontes et al., 2004; Simillion et al., 2004; Yu et al., 2005; Tuskan et al., 2006) and yeast (Seoighe and Wolfe, 1998; Fischer et al., 2006), in which chromosome rearrangements frequently occurred after WGDs.
Neither multivalent association nor pairing between homoeologous chromosomes was observed in meiosis in X. laevis (Tymowska, 1991), which implies that an ancient allotetraploid genome of X. laevis returned to a genetically diploid state through the process of diploidization of allopolyploid genomes. The present results consequently suggest that the loss of duplicated genes and chromosomal rearrangements may not have been essential for diploidization of the allotetraploid genome after WGD in X. laevis. In high-polyploid Xenopus species with 72 and 108 chromosomes, a few multivalents have been observed at the first meiotic metaphase of some spermatocytes in X. vestitus, X. wittei, X. amieti (2n=72) and X. ruwenzoriensis (2n=108), suggesting that higher polyploidization may have been the most recent event in the Xenopus lineage and diploidization has yet not been fully accomplished (Tymowska and Fischberg, 1973; Tymowska, 1991).
In this study, we constructed a comparative cytogenetic map of the allotetraploid species X. laevis. The genetic linkage and order of genes have been highly conserved between X. tropicalis and X. laevis chromosomes and also between homoeologous chromosomes of X. laevis, and WGD-derived duplicated genes have been mostly retained in homoeologous chromosomes of X. laevis. These results collectively suggest that inter- and intrachromosomal rearrangements and loss of duplicated genes have occurred less frequently in the lineage of X. laevis after allotetraploidization. Whole-genome sequencing of X. laevis and comparative chromosome mapping of other polyploid Xenopus species help us to better understand the process and mechanism of genome evolution after WGD.
Data archiving
There were no data to deposit.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (No. 23113004) and a Grant-in-Aid for Scientific Research (B) (No. 22370081) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
The authors declare no conflict of interest.
References
- Allendorf FW, Thorgaard GH.1984Tetraploidy and the evolution of salmonid fishesIn: Turner BJ (ed.)Evolutionary Genetics of Fishes Plenum Press: New York; 1–53. [Google Scholar]
- Bisbee CA, Baker MA, Wilson AC, Haji-Azumi I, Fischberg M. Albumin phylogeny for clawed frogs (Xenopus) Science. 1977;195:785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Bowers JE, Chapman BA, Rong J, Paterson AH. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 2003;422:433–438. doi: 10.1038/nature01521. [DOI] [PubMed] [Google Scholar]
- Chain FJJ, Evans BJ. Multiple mechanisms promote the retained expression of gene duplicates in the tetraploid frog Xenopus laevis. PLoS Genet. 2006;2:e56. doi: 10.1371/journal.pgen.0020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzmann RG, Davidson EA, Ferguson MM, Gharbi K, Koop BF, Hoyheim B, et al. Distribution of ancestral proto-Actinopterygian chromosome arms within the genomes of 4R-derivative salmonid fishes (Rainbow trout and Atlantic salmon) BMC Genomics. 2008;9:557. doi: 10.1186/1471-2164-9-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes R. Xenopus from the Palaeocene of Brazil and its zoogeographic importance. Nature. 1975;254:48–50. [Google Scholar]
- Evans BJ, Kelley DB, Tinsley RC, Melnick DJ, Cannatella DC. A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol Phylogenet Evol. 2004;33:197–213. doi: 10.1016/j.ympev.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Evans BJ. Genome evolution and speciation genetics of clawed frogs (Xenopus and Silurana) Front Biosci. 2008;13:4687–4706. doi: 10.2741/3033. [DOI] [PubMed] [Google Scholar]
- Fischer G, Rocha EP, Brunet F, Vergassola M, Dujon B. Highly variable rates of genome rearrangements between hemiascomycetous yeast lineages. PLoS Genet. 2006;2:e32. doi: 10.1371/journal.pgen.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyomard R, Boussaha M, Krieg F, Hervet C, Quillet E. A synthetic rainbow trout linkage map provides new insights into the salmonid whole genome duplication and the conservation of synteny among teleosts. BMC Genet. 2012;13:15. doi: 10.1186/1471-2156-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten U, Khokha MK, Grammer TC, Harland RM, Richardson P, Rokhsar DS. Accelerated gene evolution and subfunctionalization in the pseudotetraploid frog Xenopus laevis. BMC Biol. 2007;5:31. doi: 10.1186/1741-7007-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, et al. The genome of the western clawed frog Xenopus tropicalis. Science. 2010;328:633–636. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MK, Hughes AL. Evolution of duplicate genes in a tetraploid animal, Xenopus laevis. Mol Biol Evol. 1993;10:1360–1369. doi: 10.1093/oxfordjournals.molbev.a040080. [DOI] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Brunet F, Petit JL, Stange-Thomann N, Mauceli E, et al. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature. 2004;431:946–957. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- Kasahara M. The 2R hypothesis: an update. Curr Opin Immunol. 2007;19:547–552. doi: 10.1016/j.coi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kasahara M, Naruse K, Sasaki S, Nakatani Y, Qu W, Ahsan B, et al. The medaka draft genome and insights into vertebrate genome evolution. Nature. 2007;447:714–719. doi: 10.1038/nature05846. [DOI] [PubMed] [Google Scholar]
- Kellis M, Birren BW, Lander ES. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature. 2004;428:617–624. doi: 10.1038/nature02424. [DOI] [PubMed] [Google Scholar]
- Kobel HR.1996Allopolyploid speciationIn: Tinsley RC, Kobel HR (eds)The Biology of Xenopus Clarendon Press: Oxford; 391–401. [Google Scholar]
- Koyano S, Ito M, Takamatsu N, Takiguchi S, Shiba T. The Xenopus Sox3 gene expressed in oocytes of early stages. Gene. 1997;188:101–107. doi: 10.1016/s0378-1119(96)00790-1. [DOI] [PubMed] [Google Scholar]
- Krylov V, Kubickova S, Rubes J, Macha J, Tlapakova T, Seifertova E, et al. Preparation of Xenopus tropicalis whole chromosome painting probes using laser microdissection and reconstruction of X. laevis tetraploid karyotype by Zoo-FISH. Chromosome Res. 2010;18:431–439. doi: 10.1007/s10577-010-9127-x. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Chapman VM. Application of fluorescence in situ hybridization in genome analysis of the mouse. Electrophoresis. 1995;16:261–272. doi: 10.1002/elps.1150160142. [DOI] [PubMed] [Google Scholar]
- Morin RD, Chang E, Petrescu A, Liao N, Griffith M, Chow W, et al. Sequencing and analysis of 10,967 full-length cDNA clones from Xenopus laevis and Xenopus tropicalis reveals post-tetraploidization transcriptome remodeling. Genome Res. 2006;16:796–803. doi: 10.1101/gr.4871006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Evolution by Gene Duplication. Springer-Verlag: Berlin; 1970. [Google Scholar]
- Otto SP. The evolutionary consequences of polyploidy. Cell. 2007;131:452–462. doi: 10.1016/j.cell.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Pontes O, Neves N, Silva M, Lewis MS, Madlung A, Comai L, et al. Chromosomal locus rearrangements are a rapid response to formation of the allotetraploid Arabidopsis suecica genome. Proc Natl Acad Sci USA. 2004;101:18240–18245. doi: 10.1073/pnas.0407258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam NH, Butts T, Ferrier DEK, Furlong RF, Hellsten U, Kawashima T, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453:1064–1071. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hashiguchi Y, Nishida M. Temporal pattern of loss/persistence of duplicate genes involved in signal transduction and metabolic pathways after teleost-specific genome duplication. BMC Evol Biol. 2009;9:127. doi: 10.1186/1471-2148-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Steinlein C. Chromosome banding in Amphibia. XVI. High-resolution replication banding patterns in Xenopus laevis. Chromosoma. 1991;101:123–132. doi: 10.1007/BF00357062. [DOI] [PubMed] [Google Scholar]
- Sémon M, Wolfe KH. Rearrangement rate following the whole-genome duplication in teleosts. Mol Biol Evol. 2007;24:860–867. doi: 10.1093/molbev/msm003. [DOI] [PubMed] [Google Scholar]
- Sémon M, Wolfe KH. Preferential subfunctionalization of slow-evolving genes after allopolyploidization in Xenopus laevis. Proc Natl Acad Sci USA. 2008;105:8333–8338. doi: 10.1073/pnas.0708705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoighe C, Wolfe KH. Extent of genomic rearrangement after genome duplication in yeast. Proc Natl Acad Sci USA. 1998;95:4447–4452. doi: 10.1073/pnas.95.8.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simillion C, Vandepoele K, Saeys Y, Van de Peer Y. Building genomic profiles for uncovering segmental homology in the twilight zone. Genome Res. 2004;14:1095–1106. doi: 10.1101/gr.2179004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Lu P, Tang K, Osborn TC. Rapid genome change in synthetic polyploids of Brassica and its implications for polyploid evolution. Proc Natl Acad Sci USA. 1995;92:7719–7723. doi: 10.1073/pnas.92.17.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- Tymowska J.1991Polyploidy and cytogenetic variation in frogs of the genus XenopusIn: Green DS, Sessions SK (eds)Amphibian Cytogenetics and Evolution Academic Press: San Diego; 259–297. [Google Scholar]
- Tymowska J, Fischberg M. Chromosome complements of the genus Xenopus. Chromosoma. 1973;44:335–342. doi: 10.1007/BF00291027. [DOI] [PubMed] [Google Scholar]
- Uno Y, Nishida C, Yoshimoto S, Ito M, Oshima Y, Yokoyama S, et al. Diversity in the origins of sex chromosomes in anurans inferred from comparative mapping of sexual differentiation genes for three species of the Raninae and Xenopodinae. Chromosome Res. 2008;16:999–1011. doi: 10.1007/s10577-008-1257-z. [DOI] [PubMed] [Google Scholar]
- Uno Y, Nishida C, Tarui H, Ishishita S, Takagi C, Nishimura O, et al. Inference of the protokaryotypes of amniotes and tetrapods and the evolutionary processes of microchromosomes from comparative gene mapping. PLoS One. 2012;7:e53027. doi: 10.1371/journal.pone.0053027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse MR, Schnable JC, Pedersen BS, Lyons E, Lisch D, Subramaniam S, et al. Following tetraploidy in maize, a short deletion mechanism removed genes preferentially from one of the two homologs. PLoS Biol. 2010;8:e1000409. doi: 10.1371/journal.pbio.1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, Nishida-Umehara C, et al. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc Natl Acad Sci USA. 2008;105:2469–2474. doi: 10.1073/pnas.0712244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wang J, Lin W, Li S, Li H, Zhou J, et al. The genomes of Oryza sativa: a history of duplications. PLoS Biol. 2005;3:e38. doi: 10.1371/journal.pbio.0030038. [DOI] [PMC free article] [PubMed] [Google Scholar]