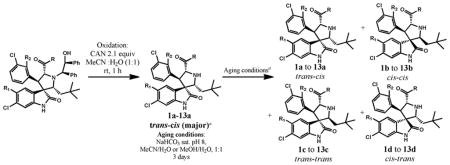
Table 1.
Isomerization of Selected Spirooxindole-Containing Compoundsa
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Compounds (a–d) | R1 | R2 | R | Yieldb(%) | Ratio after Oxidation a:b: (c+d)c | Ratio after Aging a:b: (c+d)c,d |
| 1 | 1 | H | H | NMe2 | 50e | 95:2:3 | 6:71:24 |
| 2 | 2 | H | H | NHMe | 61 | 87:5:8 | 3:71:26 |
| 3 | 3 | H | F | NMe2 | 57 | 75:19:6 | 35:46:19 |
| 4 | 4 | H | F | NHMe | 84 | 67:2:31 | 9:79:12 |
| 5 | 5 | H | F |
|
86 | 88:2:10 | 1:80:19 |
| 6 | 6 | H | F |
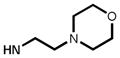
|
79 | 89:10:1 | 12:61:27 |
| 7 | 7 | H | F | OEt | 74 | 89:6:3 | 44:50:6 |
| 8 | 8f | H | F | OH | 25g | — | 41:34:25h |
| 9a | 9 | H | F | NH2 | 56 | 0:44:56 | 0:96:4 |
| 10 | 10 | F | H | NMe2 | 60 | 56:25:19 | 10:71:19i 2:88:8 |
| 11a | 11 | F | H | NHMe | 87 | 19:52:27 | 1:74:25 |
| 12 | 12 | F | H |
|
81 | 30:32:38 | 3:58:37 |
| 13a | 13 | F | H |
|
50 | 15:67:18 | 2:74:24 |
In cases of 9, 11, 12 and 13, the cis-cis isomers were obtained as the predominant products of ceric ammonium nitrate oxidation without aging (equilibrium in CH3CN and H2O at pH 8 for 3 days)
Isolated yield after flash column chromatography
Ratio determined by HPLC analysis; assignments were not made for c and d;
The combined yield of a to d in the aging step was determined from the yield in the oxidation step;
Yield after HPLC separation;
Compound 8a-8d was obtained from the hydrolysis by LiOH-THF-H2O of 7a;
Hydrolysis yield;
Starting from pure 8a;
Ratio after crude product was allowed to stand in MeOH for 2 h.
