Abstract
Background and Aims: Although the use of the laser in medical applications has increased dramatically during the last three decades, it is significant that during the last few years non-laser light sources have gained prominence in photomedicine and photosurgery, particularly the use of light-emitting diodes (LEDs) and intense pulsed light (IPL). The author therefore believed it was important to devise a new classification of light/tissue interactions, and that the well-accepted acronym LLLT and HLLT should now stand for low level light therapy and for high level light treatment, since the ‘L’ in ‘laser’, LED and IPL stands for ‘light’.
Rationale: The author herein presents a classification, which is based on the level of reaction induced by the light incident on tissue, rather than being based on the system used to deliver the light energy. When the level of tissue reactivity to light of very low incident power and energy densities is well below the cells' damage threshold, so that instead of being damaged the cells are directly activated by the low incident photon density, the changes in the irradiated tissue are photoactivative and reversible: the author hereafter refers to this group of reactions as low level light therapy (LLLT). When the level of tissue reactivity to light of very high incident power and energy densities is over the cells' damage threshold, so that the cells are directly destroyed, the changes in the irradiated tissue are photodestructive and irreversible: the author hereafter refers to this group of reactions as high level light treatment (HLLT). For levels of tissue reaction intermediate to HLLT and LLLT, the author suggests the new term, medium level light treatment (MLLT), as described in detail herein.
Conclusions: When the new classification system of light treatment (LT) is understood and used, the author feels this offers an accurate and simple method of classifying light/tissue reactions by the therapeutic reaction itself, rather than by the light source, laser, LED, IPL system or other, used to produce the reaction.
Keywords: new classification, light treatment, low level light therapy, LLLT, HLLT, MLLT, light apple
Introduction
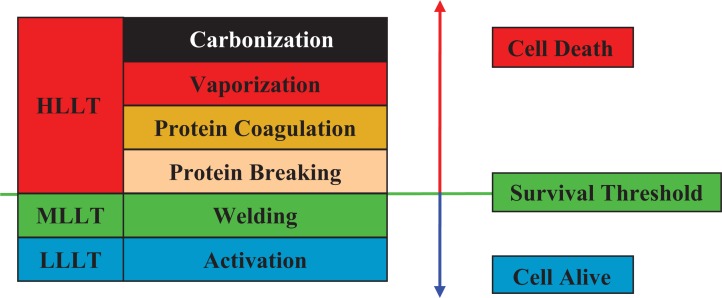
The author has previously presented his general classification of laser/tissue reactions into high-level laser treatment (HLLT, laser surgery), medium laser treatment (MLLT, laser welding) and low level laser therapy (LLLT, laser therapy),1–3) where the ‘level’ in these terms represented the level of reaction of the target cells to the incident laser energy (Figure 1). In HLLT the level of reaction was high, resulting in photodestruction and irreversible, mostly photothermally-mediated, changes to the tissue architecture. These ranged from carbonization to protein breaking, used in surgery, with an approximate temperature range from around 48°C up to 100°C and beyond.
Figure 1:
Photothermal reaction, survival threshold and relevant revised Ohshiro classification. HLLT & MLLT, high and medium level light treatment; LLLT, low level light treatment
In MLLT the level of reaction was intermediate, with a temperature range of >40°C up to around 48°C. Temperatures could possibly go higher, but only over shorter periods. Some cells in the upper range were partially thermally damaged, but left alive, whereas heat-labile bonds in the dermal matrix, such as the H2 bonds between collagen fibrils and fibers, were temporarily denatured, allowing the collagen fibers to drift apart and to migrate from their original position, floating in the dermal ground substance. When the skin temperature returned to normal, the H2 bonds renatured, but by now they were binding different fibers than before. This is the basis of tissue welding.
In Ohshiro's original classification of LLLT, the level of tissue reaction to the incident laser energy was low, below the damage threshold of the target cells (Figure 1),4,5) and resulted in photobioactivation of the target cells through a direct light-cell energy exchange. This produced a range of beneficial therapeutic effects, including enhanced wound healing, pain attenuation and accelerated repair to soft and hard tissue injuries.
However, in the past decade, a new generation of non-laser light sources, particularly clinically-useful light-emitting diodes (LEDs), has attracted a great deal of attention and has been used with clinical success in a large variety of phototherapeutic fields. Smith has subsequently argued that ‘Laser (and LED) therapy’ is phototherapy.6) The author therefore agrees that his original classification of laser treatment is now out of date, since the ‘L’ should now stand for ‘light’ rather than ‘laser’. Accordingly, he has put together the New Ohshiro Classification of Low Level Light Therapy (LLLT), which is presented in this article. The author also points out that the ‘L’ in HLLT and MLLT also stands for ‘light’ and although, it is extremely difficult to reach the necessary spectral irradiance to induce photodamage in tissue with LEDs, IPL can achieve such photodamage so HLLT and MLLT are not limited to describing laser reactions in tissue. Table 1 summarizes the three main levels of the new Ohshiro classification.
Table 1: Ohshiro's Classification of Single-System Light Treatment (SiLT).
| Single-System Light Treatment..................: SiLT |
| 1. Pure LT ..............................................: PuLT |
| 1-1. Activation LT (LLLT) ..........................: AcLT |
| 2. Auto-simultaneous LT ........................: AsLT |
| 2-1. Welding LT (MLLT) ............................: WeLT |
| 2-2. Protein Breaking LT (HLLT) ...............: PbLT |
| 2-3. Protein Coagulational LT (HLLT) .......: PcLT |
| 2-4. Vaporizational LT (HLLT) ..................: VaLT |
| 2-5. Carbonizational LT (HLLT) ................: CaLT |
Certain examples of the new Ohshiro LLLT classifications can also be applied to HLLT and MLLT, although that is beyond the scope of this present study. For this information, readers are advised to refer to Ohshiro's original effect-based classifications.4) Some of the illustrations in this article are based on Ohshiro's original ‘laser apple’ concept,7) which was so termed because of the typical apple-like scattering pattern of a laser beam in the target (Figure 2).
Figure 2:
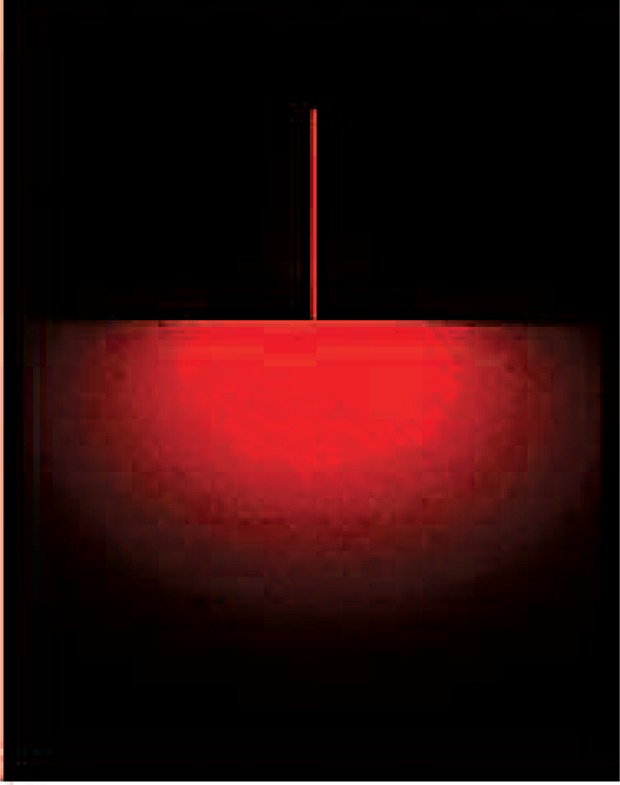
Birth of the laser apple: scattering and intensity pattern of a 630 nm HeNe beam in an acrylic target from the lateral side of the plate
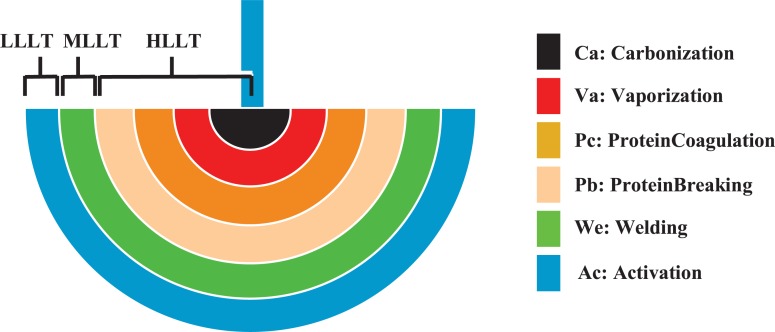
Of course that concept must also be re-termed the ‘Ohshiro light apple’, including in this case the photosurgical reactions, since the L in LLLT, MLLT and HLLT in all cases stands for light. Figure 3 shows the Ohshiro light apple and schematically demonstrates the light scatter in tissue and the entire range of tissue bioreactions which can occur with a focused surgical laser beam, such as the 10.6 µm CO2.
Figure 3:
The revised Ohshiro compound light apple, modified from the original Ohshiro laser apple, showing the range of bioreactions (denoted by different light apples, namely the AcApple, WeApple, PbApple, PcApple, VaApple and CaApple), associated temperatures and light scatter in tissue from a surgical laser beam. Note that the divisions are not cut and dried, and there will always be some overlap between each stage and the next, and that other nonthermal HLLT reactions may also occur, such as photoacoustic and photo-osmotic effects.
Light Classification
Under the modified Ohshiro system, Single-system Light Treatment falls into three main classifications: low level light therapy, medium level light treatment (MLLT) and high level light treatment (HLLT) (Table 1), and these will now be explained in detail.
As the name suggests, single-system light treatment (SiLT) is used to describe the use of a single light source (laser, LED, IPL or other non-laser source) used on its own in photomedicine or photosurgery. SiLT contains two sub-sets, pure LT (PuLT) and auto-simultaneous LT (AsLT) (Table 2 and Figure 3).
Table 2. Ohshiro's classification of light treatment & light apples.
| Description | Light Apple Name∗ |
| low level light therapy (LLLT) | AcApple |
| medium level light treatment (MLLT) | WeApple |
| high level light treatment (HLLT) | PbApple, PcApple, VaApple and CaApple |
AcApple = activation apple; WeApple = welding apple; PbApple = protein breaking apple; CaApple = carbonization apple.
PuLT describes a single reaction achieved with a single light source, for example pain attenuation with an 830 nm GaAlAs diode laser light therapy system, or fibroblast collagenesis activated with a 633 nm LED array.
AsLT is used to describe two or more simultaneous reactions achieved with the one laser or light source. In photosurgery, we might see cellular photobioactivation occurring at the periphery of the HLLT and MLLT range of reactions associated with a photo-surgical system (Table 2 and Figure 3: CaApple, VaApple, PcApple and WeApple). In the case of the PuLT and the peripheral zone of AsLT, we might see blood flow enhanced through cytokine synthesis, at the same time as activation of the descending inhibitory pathway through photoactivation of the parasympathetic nervous system.
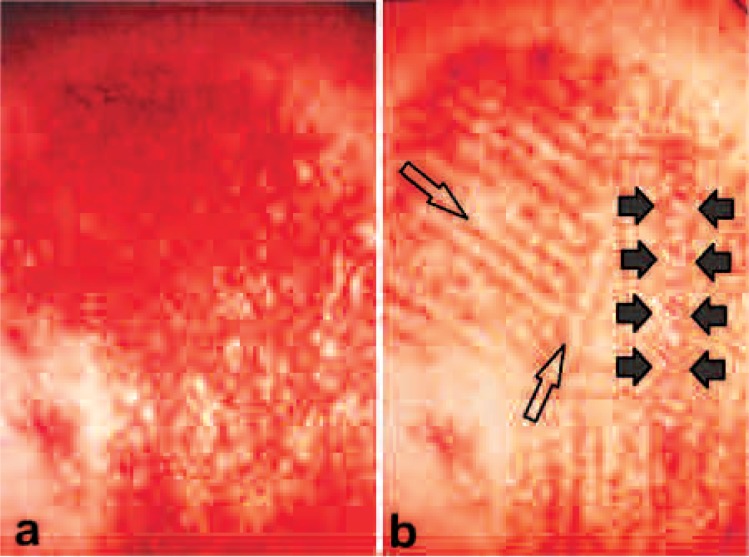
It was in fact AsLT occurring concurrently with laser surgery that drew the author's attention to laser therapy. The early adopters of the laser noted that, when the same operation was performed with laser as had been performed with the conventional scalpel, there was less postoperative pain and less inflammation associated with the laser operation. It was first suggested that the pure photothermal effect of the laser was responsible for this phenomenon, but it soon became clear that it was the ‘L’ in laser, ‘light’, that was responsible, and made laser surgery unique. In the case of a comparison between the use of needle electrolysis and laser to treat a hemangioma simplex (HS) lesion, both approaches use heat to obtain the clinical effect. Figure 4a shows an HS lesion which had been partially treated with needle electrolysis (electrocoagulation). Not only had the electrocoagulation (pure heat reaction) not removed the abnormal color of the lesion, it had left multiple tiny white millia-like scarring. The author applied the 1.5 W, 488 nm and 514.5 nm continuous wave argon laser in his photothermal HLLT zebra technique, whereby narrow bands (typically 2–4 mm) of the lesion were first treated with the argon laser leaving intermediate equal bands of untreated tissue between the treated bands. Once the treated bands had healed, with improved color, the untreated bands were then treated. In Figure 4b the treated areas in the HS are clearly returning to normal skin color compared with the untreated areas, but the abnormal configuration (i.e., scarring) has also been remedied. Even though the argon laser and electrocoagulation treatment both depended on delivering a heat effect, only the additional influence of the light in the laser treatment enabled a good result, compared with the heat-only effect of electrocoagulation. This led the author to explore the dedicated application of photobioactivation and low level laser therapy, inspired by the exciting reports on phototherapy that were appearing from the Hungarian researcher and clinician, Endrè Mester, the ‘Godfather’ of phototherapy.7)
Figure 4:
Hemangioma simplex lesion. a: findings on presenting at the author's clinic. The lesion had been treated elsewhere with needle electrocautery. Note the small white scars and failure to remove the color. b: Lesion after first set of treatments with the argon laser (see text for details). Note the presence of both color and small scars in the zone between the solid arrows, compared with removal of both color and abnormal configuration in the treated areas (examples shown with open arrows)
Discussion
The question of the correct use of terminology and its importance in the accurate reporting of clinical and surgical trials and experiments has been dealt with in some detail in the literature,8) and is a favorite subject of the author. Without accurate reporting and the use of appropriate terminology, other researchers will be unable accurately to repeat clinical trials or experimental studies, to corroborate data and validate findings. This leads to lack of scientific acceptance of the specific study or trial in particular, and of the laser in surgery and medicine in general. Therapeutic applications of the laser and non-laser phototherapy tend to have more problems along these lines than surgical applications, mostly because laser surgery has a much longer history and is used in many more specialities than is the case with low level light therapy.
It is worthy of note here that our terminology also needs to be updated regarding the description of laser therapy systems when reporting their use. Under the former classification, the term of ‘the 830 nm GaAlAs diode LLLT’ was quite acceptable, because we were talking about ‘low level laser therapy’. Now, however, under the revised Ohshiro classification, the correct term is low level light therapy, so when referring to a laser system we have to add the word ‘laser’ for clarification, as follows: ‘830 nm GaAlAs diode laser LLLT’. For an LED source, we can simply refer to ‘830 nm LED LLLT’.
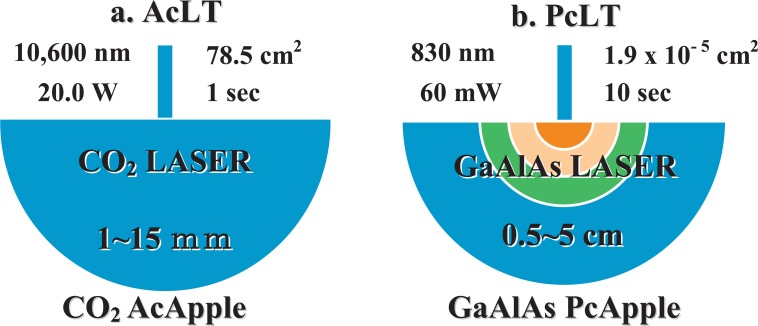
An example of current terminology which lacks a scientific base, is the continuing use of such terms as ‘low power laser’ and ‘high power laser’, or the marketing-driven and even more unscientific ‘soft laser’ and ‘hard laser’ to refer to therapeutic laser systems and surgical laser systems, respectively.8) The author is of the opinion that, while there may certainly be lasers with low output powers and lasers with high output powers, they can actually both be used to produce an entire range of tissue reactions. This is illustrated in Figure 5, where a laser normally associated with surgery and HLLT, a 20 W, 10600 nm CO2 laser, is applied with a 10 cm diameter spot size to produce an LLLT Ac-Apple, while a laser normally associated with diode laser LLLT, a 60 mW, 830 nm GaAlAs system, is producing an HLLT VaApple or PcApple with a highly focused beam. In this case the ‘high power laser’ is delivering incident power densities associated with laser therapy, while the so-called ‘low power laser’ is vaporizing and coagulating the target tissue.
Figure 5:
The laser apples at work, showing how neither the output power of the light source nor the light source itself should be used to classify the treatment. In Fig 6a, a defocused 20 W, 10,600 nm CO2 laser (10 cm diameter spot) is delivering around 250 mW/cm2, a true LLLT power density, whereas the 60 mW, 830 nm GaAlAs diode laser is delivering over 3,000 W/cm2, an HLLT capable beam.
Although the author has classified MLLT and HLLT as medium- and high level light treatment, thereby including IPL treatments in these categories, in actual clinical practice it is impossible at present to treat tissue using an IPL system with the precision and selectivity of laser energy. For the moment, therefore, HLLT and MLLT really only encompass procedures carried out with a laser because of both the potential tissue or cell-selectivity, and controllability of the actual level of tissue reaction, ranging from coagulation and vaporization down to the higher levels of protein denaturation. However, auto-simultaneous light therapy (AsLT) also occurs together with these destructive reactions, so the author defends classifying the second ‘L’ in HLLT and MLLT as ‘light’.
The most important point the author wishes to stress is that the level of tissue reaction should be used to classify laser systems, and not any aspect of the hardware or its power level. A laser, LED or IPL system is merely the device which is capable of generating a beam of light energy. It is the reaction with the tissue which gives this beam of special energy its clinical utility, whether for laser surgery or for light therapy. That is why the author has developed this new classification system based on the tissue reaction. Ohshiro also previously designed the ‘laser apple’ concept, now of course renamed as the Ohshiro ‘light apple’, so that a single pictorial representation is capable of giving all salient characteristics of a laser beam which not only includes all the physical parameters associated with the laser itself but also goes further and graphically represents the laser tissue reactions and the beam spread and penetration.
References
- Ohshiro T, Calderhead RG. (1988): Low Level Laser Therapy: A Practical Introduction. John Wiley & Sons, Chichester, UK [Google Scholar]
- Ohshiro T.(1991): Low Reactive Level Laser Therapy: Practical Application. John Wiley & Sons, Chichester, UK [Google Scholar]
- Ohshiro T. (1995): Laser Treatment for Naevi. John Wiley & Sons, Chichester, UK [Google Scholar]
- Ohshiro T. (1996): A new effect-based classification of laser applications in surgery and medicine. Laser Therapy, 233–240 [Google Scholar]
- Ohshiro T. (2005): Auto-simultaneous laser treatment: a new effect-based classification. Laser Therapy, 14: 11–17 [Google Scholar]
- Smith KC. (2005): Laser (and LED) therapy is phototherapy. Photomed Laser Surg. 23: 78–80 [DOI] [PubMed] [Google Scholar]
- Ohshiro T. (1996): The laser apple: a new graphic representation of medical laser applications. Laser Therapy, 8: 185–190 [Google Scholar]
- Ohshiro T. (2008): A tribute to the late Endré Mester, the ‘godfather’ of phototherapy. Laser Therapy, 17: 5–7 [Google Scholar]
- Calderhead RG. (1991): Watts a joule: on the importance of accurate and correct reporting of laser parameters in low reactive-level laser therapy and photobioactivation research. Laser Therapy, 3: 177–182 [Google Scholar]