Abstract
T cell homeostasis and survival is dependent on interleukin-7 (IL-7). Immune activation, however, downregulates IL-7 receptor expression on T cells so that T cell survival during activation must be maintained independently of IL-7. The pro-inflammatory cytokine IL-6 shares common signaling pathways with IL-7 and can promote T cell survival in vitro. But whether IL-6 promotes T cell survival and homeostasis in vivo is not clear. Notably, IL-6 overexpression results in massive plasmacytosis and autoimmunity so that an IL-6 effect on in vivo T cell survival has remained untested. To overcome this limitation, here we generated IL-6 transgenic mice on an immunoglobulin heavy chain (IgH) deficient background which rendered them B cell deficient. Notably, such IgHKOIL6Tg mice were free of any signs of inflammation or autoimmunity and remained healthy throughout the course of analysis. In these mice, we found that IL-6 overexpression significantly increased peripheral T cell numbers, but importantly without increasing thymopoiesis. Moreover, IL-6 signaled T cells maintained their naïve phenotype and did not express activation/memory markers, suggesting that increased T cell numbers were due to increased T cell survival and not because of expansion of activated T cells. Mechanistically, we found that IL-6 signaling induced expression of pro-survival factors Mcl-1 and Pim-1/-2 but not Bcl-2. Thus, IL-6 is a T cell homeostatic cytokine that expands T cell space and can maintain the naïve T cell pool.
Keywords: Apoptosis, Cytokine, Survival, Proliferation, Thymus
1. Introduction
T cells are generated in the thymus and then migrate into peripheral tissue for immune surveillance and protection. Maintaining T cells in the periphery is referred to as T cell homeostasis, and cytokines play essential roles in this process [1]. Specifically, interleukin-7 (IL-7) is a non-redundant cytokine in T cell homeostasis, and in vivo availability of IL-7 sets the size of the peripheral T cell pool [2–4]. IL-7 sustains T cell survival by providing anti-apoptotic signals, inhibiting pro-apoptotic activities, and promoting cell metabolism. To do so, IL-7 signaling upregulates Bcl-2, inhibits Bax and Bad, and induces expression of glucose transporter-1 [5–8]. Collectively, IL-7 is an essential pro-survival signal that maintains the size and composition of the T cell pool under steady state conditions.
IL-7 is a member of the common γ-chain (γc) cytokine family that also includes IL-2, IL-4, IL-9, IL-15 and IL-21 [9]. γc cytokines share the γc receptor for ligand binding and signaling, and have common characteristics in their signaling pathways. All γc cytokines, including IL-7, induce activation of receptor bound Janus kinases (JAK) which leads to phosphorylation and nuclear translocation of STAT molecules. PI3-kinase/Akt activation is another major pathway induced by all γc cytokines [10–12]. Because of such similarities in their downstream signaling effects, it has been a longstanding question what makes IL-7 unique in its ability to drive T cell homeostasis. Also, it has remained unclear if cytokines other than IL-7 can act redundantly to IL-7 in T cell homeostasis. Interestingly, overexpression of most γc cytokines failed to maintain naïve T cell homeostasis in vivo [13–16]. Transgenic expression of IL-2 or IL-4 resulted in severe inflammation and loss of naïve T cells due to aberrant T cell activation [15, 16]. IL-15 transgenic mice showed dramatic expansion and accumulation of memory phenotype CD8 T cells with minimal contribution to naïve CD8 T cell survival [14]. IL-21 overexpression increased the CD8 memory T cell pool concomitant to significantly reduced naïve T cell numbers [13]. Thus so far, no γc cytokine other than IL-7 has been found to promote naïve T cell homeostasis.
A unique feature of IL-7 signaling is downregulating expression of its own receptor [17, 18]. We have previously shown that this mechanism maximizes the availability of limited in vivo IL -7 and that it increases the size of the naive T cell pool [18]. On the other hand, signaling of other γc cytokines upregulates expression of their own receptors, resulting in further reinforcement of γc cytokine signaling and expansion of memory/activated phenotype cells, presumably at the expense of naïve T cells [19, 20]. As such, downregulating expression of its own receptor contributes to the molecular basis of a homeostatic cytokine.
In the current study, we made the serendipitous finding that the non-γc cytokine IL-6 also downregulates expression of its own receptor. IL-6 is a pro-inflammatory cytokine that is produced by many cell types, including stromal cells, endothelial cells, and lymphocytes [21]. IL-6 is largely known for its inflammatory effects and its involvement in cancer and autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and Crohn’s disease [22, 23]. Consequently, IL-6 deficiency ameliorates a series of experimental autoimmune diseases, including induction of Experimental Autoimmune Encephalomyelitis (EAE) [24, 25], collagen-induced arthritis [26], and colitis [27]. Along this line, recent studies revealed a role for IL-6 on the generation of the pro-inflammatory T cell subset, Th17 cells [28–30], and its suppressive effect on FoxP3+ regulatory CD4 T cells [28, 31]. Thus, IL-6 signaling is a central component of a pro-inflammatory response. IL-6 signals presumably through a hexameric complex composed of two heterotrimers of IL-6, IL-6Rα, and gp130 [32]. Previous studies have shown that IL-6 can promote T cell survival in vitro utilizing the same pathways as IL-7 by activating JAK/STAT and PI3-K/Akt, but whether IL-6 can act as a homeostatic cytokine in vivo has remained unresolved [33–35]. Addressing this question in vivo is further complicated because IL-6 overexpression induced severe plasmacytosis so that IL-6 transgenic mice (IL6Tg) developed massive lymphoid organ infiltrates of plasma cells, resulting in premature death due to glomerulonephritis-induced renal failure [36, 37].
To circumvent this problem, here we generated B cell-deficient IL6Tg mice by introducing the IL6Tg onto an immunoglobulin heavy chain (IgH) deficient (IgHKO) background. Such B cell-deficient IgHKOIL6Tg mice survived more than one year without developing any immunopathology and autoimmune diseases. Notably, IL-6 overexpression did not affect T cell development in the thymus and did not induce activation of mature T cells in the periphery. In fact, composition of the peripheral T cell compartment of IgHKOIL6Tg mice was in distinguishable to that of control IgHKO mice. However, naïve T cell numbers in IgHKOIL6Tg mice were significantly increased, and T cells expressed increased levels of survival factors such as Pim-1/2 and Mcl-1. Collectively, these results identify IL-6 as a novel homeostatic cytokine for T cells that can expand the peripheral T cell space and potentially contributes to maintaining the naïve T cell pool under inflammatory conditions.
2. Materials and methods
2.1 Mice
C57BL/6 (B6) mice were obtained from the Frederick Cancer Research and Development Center, Frederick, MD. IgH-deficient (IgHKO) mice and IL-6 transgenic (IL6Tg) mice, expressing human IL-6 under the control of the MHC-I promoter, have been previously described [36, 37] and purchased from the Jackson Laboratory (Bar Harbor, ME). Animal experiments were approved by the NCI Animal Care and Use Committee, and all mice were cared for in accordance with NIH guidelines.
2.2 Cell isolation and cell culture
Lymph node (LN) T cells from B6 mice were isolated by depleting B cells with anti-mouse IgG beads (Qiagen). LN T cells were cultured in media or recombinant murine IL-6 (10 ng/mL; PeproTech) and IL-7 (10 ng/mL; PeproTech) for RNA isolation or viability assay. CD4+ LN T cells from IgHKO and IgHKOIL6Tg mice were isolated by depleting CD8+ cells with anti-CD8 antibody (2.43) and anti-rat IgG BioMag beads (Qiagen).
2.3 Quantitative reverse transcription PCR
Total RNA was isolated with the RNeasy kit (Qiagen). RNA was reverse transcribed into cDNA by oligo(dT) priming with the QuantiTect Reverse transcription kit (Qiagen). Quantitative reverse transcription PCR (qRT-PCR) was performed with an ABI PRISM 7900HT Sequence Detection System and the QuantiTect SYBR Green detection system (Qiagen) with the primers for the following molecules: IL-6Rα (F: 5′-GCAGGAATC CTCTGGAACCC-3′, R: 5′-CAGAAGGAAGGTCGGCTTCA-3′), IL-7Rα (F: 5′-CACACAAGAACAACAATCCCACA-3′, R: 5′-GATCCCATCCTCCTTGATTCTTG -3′), Bcl-2 (F: 5′-TGTAAATTGCCGAGAAGAAGGG-3′, R: 5′-TCCCCGTTGGCATGAGAT-3′). Bcl-xL (F: 5′-GCGGCTGGGACACTTTTG-3′, R: 5′-ACTTCCGACTGAAGAGTGAGCC- 3′), Mcl-1 (F: 5′-AGACGGCCTTCCAGGGC -3′, R: 5′-CCAGTCCCGTTTCGTCCTT-3′) Pim-1 (F: 5′-ACCTGAGCCGCGGCGAAATC-3′, R: 5′-GCCGTGGTAGCGATGGTAGCG-3′), Pim-2 (F: 5′-CACCGTCTTCGCGGGACACC-3′, R: 5′-CCACCTTCCACAGCAGCGCA- 3′). Gene expression values were normalized to those of HPRT (F: 5′-GCGATGATGAACCAGGTTATGA-3′, R: 5′-ACAATGTGATGGCCTCCCAT - 3′) in the same sample.
2.4 Flow cytometry
Single cell suspensions were prepared from thymus or LN, and then stained and analyzed on an LSRII, FACSAria or FACSCalibur (BD Biosciences). Dead cells were excluded by forward light scatter gating and propidium iodide (PI) staining for viability assays and for phenotyping. Annexin V/PI staining was performed according to the manufacturer’s instructions (BD Biosciences). Total caspase activity was assessed using a CaspGLOW fluorescein active caspase staining kit (eBioscience). Antibodies with the following specificities were used for staining: CD4 (GK1.5 and RM4.5), CD25 (PC61.5), CD8α (53-6-7), TCRβ (H57-597), IL-7Rα (A7R34), Ki-67, and Foxp3 (FJK-16s; all from eBiosciences), CD69 (H1.2F3), IL-6Rα (M5), CD44 (IM7), CD62L (MEL-14) and pSTAT3 (pY705; all from BD Biosciences), Helios (22F6) and CD122 (5H4; all from BioLegend). STAT3 phosphorylation was determined by methanol/acetone fixation for 30 min after IL-6 stimulation [38]. For intra-nuclear Ki-67, Foxp3 and Helios staining, cells were fixed and permeabilized with Foxp3 fixation/permeabilization buffer (eBiosciences). Data were analyzed using software designed by the Division of Computer Research and Technology at the NIH.
2.5 TREC analysis
Cell lysates were prepared from either 3 × 106 LN cells using DNAzol (Molecular Research Center, Inc.), or 5 × 106 LN cells using the QIAmp Blood Mini Kit (QIAgen), as per manufacturer’s instructions. Isolated DNA from cell lysates was then amplified using the TaqMan real-time PCR assay (Applied Biosystems) specific for the mδRec primer (5′-GGGCACACAGCAGCTGTG-3′), the ψJα primer (5′-GCAGGTTTTTGTAAAGGTGCTCA), and the mδRec-ψJα fluorescent probe (5′-FAM-CACAAGCACCTGCACCCTGTGCA-TAMRA-3′). Primers for the single-copy CD8β gene include the forward primer (5′-CAGGACCCCAAGGACAAGTACT-3′), the reverse primer (5′-CACTTTCACCATACAAAACTCCTTTG) and probe (5′-FAM-TGAGTTCCTGGCCTCCTGGAGTTCTTC-TAMRA-3′). Primers were obtained from Invitrogen, and probes were ordered from Eurofins MWG Operon. Standard curves were generated per experiment by cloning the signal joint mδRec-ψJα TREC or the CD8β gene into a pCR-XL TOPO vector. TREC copy numbers were determined for 50,000 cells, normalized to CD8β, and then analyzed relative to littermate control.
2.6 IL-6 ELISA
Serum samples were obtained from IgHKO and IgHKOIL6Tg mice. Serum human IL-6 levels were determined by ELISA Ready-SET-Go kit according to manufacturer’s instructions (eBioscience).
2.7 Immunoblotting
T cells ere were lysed in Cell Lytic-M (Sigma) supplemented with protease inhibitors (Roche). Whole-cell lysates were electrophoresed in 10% Tri/glycine gels and transferred to PVDF membranes (Invitrogen). Blots were incubated with anti-Mcl-1 (Rockland Immunochemicals) or anti-actin antibodies (Santa Cruz Biotechnology), and detected using horseradish peroxidase conjugated anti-rabbit IgG antibodies. Reactivity was visualized by enhanced chemiluminescence (Pierce).
2.8 BrdU incorporation assay
IgHKO and IgHKOIL6Tg mice were given an initial intraperitoneal injection of BrdU (1 mg dissolved in PBS) and then kept on BrdU containing drinking water (1mg/ml) for 3 days. Thymocytes and LN T cells were first stained with anti-CD4, anti-CD8α, and anti-TCRβ antibodies, and then fixed and permeabilized with Cytofix/Cytoperm and Cytofix/Cytoperm Plus for intranuclear anti-BrdU staining according to the manufacturer’s protocol (BD Biosciences).
2.9 Statistical analysis
Data are shown as mean ± SEM. Two-tailed Student’s t-test was used to calculate P-values for all experiments except in the calculation of MFI in which paired t-test was used. A value of P ≤0.05 was considered statistically significant.
3. Results
3.1 IL-6 promotes T cell survival in vitro
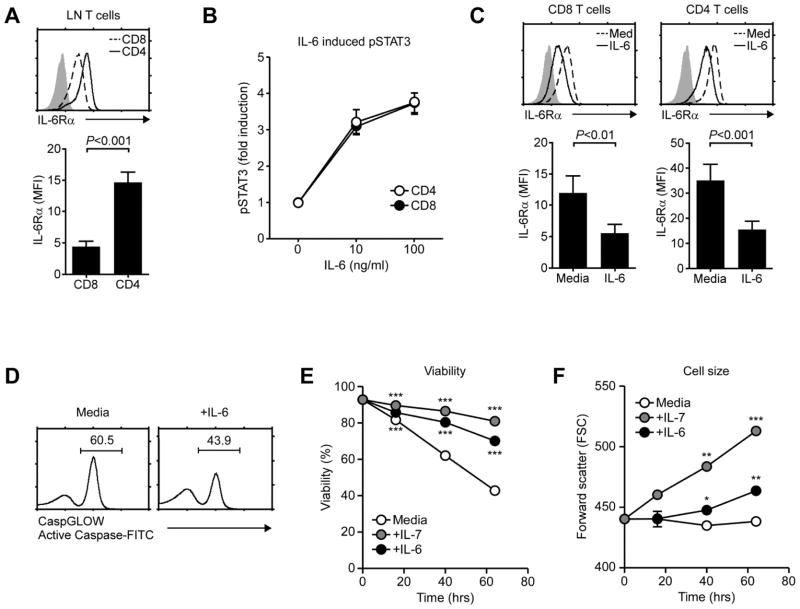
To assess a role for IL-6 in T cell homeostasis, first we analyzed IL-6Rα expression on T cells under steady state conditions. All T cells expressed IL-6Rα, but CD4 T cells expressed significantly higher levels of IL-6Rα than CD8 T cells (Fig. 1A). Such difference in IL-6Rα levels, however, did not result in distinct IL-6 signaling as IL-6-induced STAT3 phosphorylation in vitro was identical between CD4 and CD8 T cells (Fig. 1B). Whether such is also the case in vivo remains untested. Nevertheless, these results suggest that both CD4 and CD8 T cells are IL-6 signaling competent.
Fig. 1. IL-6 promotes T cell survival in vitro.
(A) IL-6Rα expression on LN T cells. IL-6Rα levels were determined on freshly isolated CD4 and CD8 LN T cells (top) and quantified in mean fluorescence intensities (MFI) (bottom). Results show the mean +/− SEM of five independent experiments.
(B) IL-6 signaling in CD4 and CD8 T cells. Phospho-STAT3 (pSTAT3) contents upon IL-6 signaling were assessed in wildtype CD4 and CD8 T cells by intracellular staining. Data show the summary of eight independent experiments.
(C) IL-6Rα levels on IL-6 stimulated LN T cells. LN T cells were incubated overnight with IL-6 or medium alone. Next day, surface IL-6Rα levels were assessed on CD4 and CD8 T cells (top). Bar graphs show the mean +/− SEM of IL-6Rα levels from seven independent experiments.
(D) Total caspase activity upon IL-6 stimulation. Purified LN T cells were cultured for 48 hours with IL-6 or medium alone. Caspase activities were assessed by incubation with FITC-conjugated caspase inhibitor peptides. Histograms show representative results from three independent experiments.
(E) Survival curve of IL-6 treated T cells. Purified LN T cells were cultured in medium, IL-7 or IL-6 for the indicated time. Cell viability was determined by propidium iodide exclusion. Data are the summary of four independent experiments. ***P<0.001, two-tailed Student’s t-test.
(F) Cell size assessment of IL-6 treated T cells. LN T cells were cultured in media, IL-7 or IL-6 for the indicated time. Cell sizes were determined by Forward Scatter (FSC) analysis. Data are the results of three independent experiments. *P<0.05; **P<0.01; ***P<0.001, two-tailed Student’s t-test.
Analysis of IL-6 stimulated CD4 and CD8 T cells showed that IL-6 induced downregulation of its own receptor (Fig. 1C). This is in contrast to T cell activating cytokines, such as IL-2 and IL-4, which upregulate expression of their own receptors [19, 20]. Thus, IL-6 signaling is more in line with homeostatic IL-7 signaling, which downregulates expression of its own receptor to maximize in vivo IL-7 availability [18]. To further determine the downstream effects of IL-6, we assessed the phenotype of IL-6 treated T cells. We found that IL-6 stimulation significantly suppressed pro-apoptotic caspase activities (Fig. 1D), and that IL-6 promoted viability and increased cell size (Fig. 1E, F, and Suppl. Fig. 1A). These results demonstrate anti-apoptotic and pro-metabolic functions of IL-6 on resting T cells.
To understand the pro-survival effect of IL-6, we analyzed mRNA contents of IL-6 treated LN T cells. We found that IL-6 upregulated expression of anti-apoptotic Mcl-1, Pim-1, and Pim-2 [39], but not of Bcl-xL and Bcl-2 (Fig. 2A). Notably, while Mcl1 expression was induced by both IL-6 and IL-7 (Fig. 2B), Bcl-2 mRNA expression was only induced by IL-7 and not by IL-6, suggesting that the pro-survival effects of IL-7 and IL-6 are distinct. Collectively, these data establish IL-6 as a pro-survival cytokine for T cells that induces expression of the pro-metabolic kinases Pim-1/2 and upregulates expression of the anti-apoptotic Mcl-1.
Fig. 2. IL-6 signaling induces expression pro-survival and pro-metabolic factors.
A) Pro-survival factor expression in IL-6 stimulated T cells. Purified LN T cells were incubated overnight in medium, IL-7 or IL-6. mRNA expression of the indicated genes were assessed by qRT-PCR. Data show the mean +/− SEM of three independent experiments.
(B) Immunoblot analysis of Mcl-1 expression in cytokine stimulated cells. Purified LN T cells were incubated with IL-6, IL-7 or in medium alone for 24 hours, and total cell lysates were probed for Mcl-1 and then reprobed for β-actin as loading control. Numbers in box indicate relative Mcl-1 expression as determined by densitometry of the bands. Blot is representative of two independent experiments.
3.2 IL-6 promotes peripheral T cell survival in vivo
To determine if IL-6 can promote T cell survival in vivo, next we analyzed T cell homeostasis in IL-6 transgenic mice (IL-6Tg). Importantly, we made IL-6Tg mice additionally B cell deficient (IgHKOIL6Tg) to prevent B cell hyperactivation and plasmacytosis as consistently observed in B cell sufficient IL-6Tg mice [36]. Such IgHKOIL6Tg mice expressed high levels of IL-6 in serum but did not show any signs of inflammation and remained tumor-free for more than 18 months (Fig. 3A and data not shown). Thymocyte development in IgHKOIL6Tg mice was normal and comparable to control IgHKO mice. Specifically, transgenic IL-6 did not affect CD4/CD8 profiles of total and TCRβ+HSAlow mature thymocytes, and overall thymocyte numbers remained the same (Fig. 3B, C, and D). Thus, IL-6 overexpression did not perturb thymopoiesis or lineage commitment.
Fig. 3. Thymocyte development in IL-6 transgenic mice.
(A) Transgenic IL-6 expression in IgHKOIL6Tg mice. Serum levels of transgenic human IL-6 were determined by ELISA. Results show the mean +/− SEM of two independent experiments with total six IgHKO and seven IgHKOIL6Tg mice.
(B) Thymocyte development in IgHKOIL6Tg mice. Contour plots show CD4/CD8 profiles of total thymocytes (top) and of TCRβ+HSAlo mature thymocytes (bottom).
(C) Thymocyte numbers in IgHKOIL6Tg mice. Total thymocyte numbers were determined in five separate experiments from seven IgHKO and nine IgHKOIL6Tg mice.
(D) CD4/CD8 lineage differentiation in IgHKOIL6Tg mice. CD4/CD8 ratio was determined from TCRβ+HSAlo mature thymocytes. Data show mean +/− SEM of four independent experiments with a total of seven IgHKO and six IgHKOIL6Tg mice.
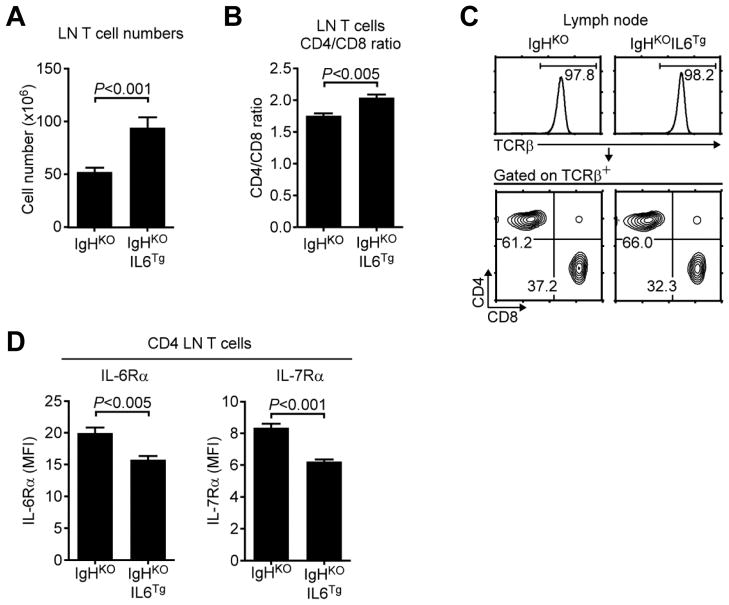
In the periphery, however, transgenic IL-6 induced a significant increase of total T cell numbers even as we did not observe increased viability in ex vivo isolated cells (Fig. 4A and Suppl. Fig. 1B). Interestingly, we also observed a modest but significant bias toward CD4 T cell survival, suggesting a preferential effect of IL-6 on CD4 lineage T cells (Fig. 4B and C). Moreover, only αβ T cells and not γδ T cells accumulated in IgHKOIL6Tg mice (Fig. 4C and Suppl. Fig. 1C), indicating that transgenic IL-6 mostly promoted survival of αβ T cells. Finally, in agreement with in vitro results (Fig. 1C), we found that in vivo IL-6 stimulation also suppressed IL-6Rα expression on IgHKOIL6Tg CD4 T cells (Fig. 4D left). Since IL-7Rα expression was also downregulated in trans by in vivo IL-6 (Fig. 4D right), these results suggest an interplay of two pro-survival cytokines that could mutually limit their consumptions and maximizes their bioavailability.
Fig. 4. IL-6 promotes peripheral T cell homeostasis.
(A) LN T cell numbers in IgHKO and IgHKOIL6Tg mice. Data show the mean +/− SEM of five independent experiments with a total of seven IgHKO and eight IgHKOIL6Tg mice.
(B) LN cell analysis of IgHKO and IgHKOIL6Tg mice. Histograms show TCRβ expression of total LN cells (top). Contour plots show CD4/CD8 profiles of TCRβ+ gated LN T cells. Results are representative of five independent experiments with seven IgHKO and eight IgHKOIL6Tg mice.
(C) CD4/CD8 ratio of LN T cells. Data show mean +/− SEM of four independent experiments with a total of seven IgHKO and six IgHKOIL6Tg mice.
(D) Cytokine receptor expression on IgHKO and IgHKOIL6Tg T cells. Surface IL-6Rα and IL-7Rα levels were quantified in MFI. Bar graphs show the mean +/− SEM of four independent experiments with seven IgHKO and six IgHKOIL6Tg mice.
3.3 T cell quiescence is maintained in IL-6 transgenic mice
IL-6 can directly induce T cell proliferation under lymphopenic conditions [40], and can indirectly induce T cell expansion by triggering excess IL-7 production in non-hematopoietic cells [41]. In both cases, IL-6 dependent T cell expansion was associated with T cell activation and autoimmunity such as colitis and rheumatoid arthritis. To examine whether IL-6 overexpression in IgHKOIL6Tg mice induced T cell expansion by T cell activation, next we assessed their T cells for activation markers. Notably, CD25 and CD69 expression were not upregulated and were not different from control IgHKO T cells (Fig. 5A). IgHKOIL6Tg mice also did not show increased accumulation of activated/memory phenotype CD62LloCD44hi CD4 T cells or CD122hiCD44hi CD8 T cells (Fig. 5B). Moreover, intracellular staining for the proliferation-associated nuclear antigen Ki-67 and in vivo BrdU labeling showed that IL-6 overexpression did not induce cell cycling and proliferation of peripheral T cells (Fig. 5C, D, and Suppl. Fig. 1D).
Fig. 5. T cell quiescence is maintained in IgHKOIL6Tg mice.
(A) Surface CD69 and CD25 expression on IgHKO and IgHKOIL6Tg T cells. Histograms are representative of three independent analyses with each one mouse.
(B) Activated/memory phenotype cells percentages in CD4 and CD8 LN T cells. Contour plots of surface CD62L and CD122 versus CD44 expression are representative of three independent analyses with each one mouse.
(C) Ki-67 intra-nuclear staining of CD4 LN T cells from IgHKO and IgHKOIL6Tg mice. Histogram is representative of and bar graph shows the mean +/− SEM from three independent experiments with each one mouse.
(D) BrdU incorporation in DN thmocytes and LN T cells from IgHKO and IgHKOIL6Tg mice. After initial i.p. injection with BrdU, mice were supplied with BrdU in drinking water for 3 days. BrdU incorporation was assessed by anti-BrdU intracellular staining. Bar graph shows the mean +/− SEM for the indicated cells from two independent experiments with two IgHKO and five IgHKOIL6Tg mice.
(E) FoxP3+ and Helios+ CD4 T cells in IgHKO and IgHKOIL6Tg mice. Contour plots are representative of three independent analyses with each one mouse.
(F) FoxP3+CD25+ CD4 Treg cell percentages in IgHKO and IgHKOIL6Tg LN cells. Data show the mean +/− SEM percentages of FoxP3+CD25+ cells among CD4 LN T cells from three independent experiments.
Along this line, we found that homeostasis of FoxP3+ and Helios+ regulatory CD4 T cells (Tregs) were not affected in IL-6 transgenic mice (Fig. 5E, F) [42, 43]. Previously, IL-6 had been reported to suppress FoxP3+ Treg cell generation [28], and reduced Treg activity was shown to result in T cell activation and autoimmunity [44]. While lower Treg cell numbers could have been a cause for greater T cell numbers in IgHKOIL6Tg mice, comparable FoxP3+CD4+ T cell percentages did not favor this possibility. Collectively, these data suggest that increased T cell number in IgHKOIL6Tg mice is not associated with T cell activation, and that transgenic IL-6 promotes T cell expansion without perturbing quiescence of the peripheral T cell pool.
3.4 IL-6 expands T cell space by increasing T cell survival and extending life span
To further establish that increased T cell numbers are mediated by increased T cell survival and independent of thymic output, we examined T cells in aged IgHKO and IgHKOIL6Tg mice (37 to 52 weeks). In aged mice, the thymus begins to atrophy and thymic output is significantly reduced [45]. Transgenic IL-6 did not reverse this phenotype, and overall thymocyte numbers and CD4/CD8 lineage choice remained comparable to control IgHKO mice (Suppl. Fig. 2A, B). Nevertheless, LN T cell numbers in aged IgHKOIL6Tg mice were still significantly increased and T cells displayed a similar phenotype as in young IgHKOIL6Tg mice (Suppl. Fig. 2C, D, E). Importantly, such increase in cell numbers was not because of preferential T cell migration to LN or tissue re-distribution of T cells since T cell numbers in the spleen was not reduced but rather increased (Fig. 6A), and it was specific to T cells because spleen NKT cell numbers were unaffected (Fig. 6B).
Fig. 6. IL-6 is an in vivo T cell survival factor.
(A) Spleen T cell numbers in aged IgHKO and IgHKOIL6Tg mice. Data show the mean +/− SEM from ten IgHKO and nine IgHKOIL6Tg mice.
(B) Spleen NKT cell numbers in aged IgHKO and IgHKOIL6Tg mice. Data show the mean +/− SEM from six IgHKO and seven IgHKOIL6Tg mice.
(C) TREC copy numbers in IgHKO and IgHKOIL6Tg T cells. TREC copy numbers were determined relative to TREC numbers in IgHKO T cells, which was set to 100. Results show the mean +/− SEM of three independent experiments.
(D) Naïve and memory phenotype T cells in aged IgHKO and IgHKOIL6Tg mice. CD62L, CD122 and CD44 expression were determined on gated CD4 and CD8 T cells of aged (37–52 weeks) mice. Data are representative of eight independent experiments with eight IgHKO and eleven IgHKOIL6Tg mice.
(E) Pro-survival factor expression in IgHKOIL6Tg T cells. mRNA content of the indicated genes were assessed in purified IgHKO and IgHKOIL6Tg CD4 T cells by RT-qPCR. Data show the mean +/− SEM of five independent experiments with a total of six IgHKO and six IgHKOIL6Tg mice.
Moreover, T cells populating the periphery of IgHKOIL6Tg mice contained decreased copy numbers of TRECs (T cell receptor excision circle) when compared to T cells from age matched IgHKOIL6Tg mice (Fig. 6C). TRECs are generated only in the thymus during T cell receptor recombination and are not degraded in the periphery. Thus, dilution of TREC numbers suggests that cells have undergone increased rounds of proliferations [46]. Upon acute proliferation, however, T cells acquire an activated/memory phenotype which is marked by increased expression of CD44 [47, 48]. Interestingly, even in aged mice (37–52 weeks), IgHKOIL6Tg T cells contained normal levels of activated/memory phenotype cells (Fig. 6D), which suggest that increased T cell numbers were possibly induced by prolonged survival and slow proliferation. In fact, in vivo IL-6 strongly induced expression of a series of pro-survival factors similar to those observed in vitro, which included Pim-1, Pim-2, and Mcl-1 (Fig. 6E). Notably, Bcl-xL expression, which was not induced by in vitro IL-6 stimulation, was now significantly induced by in vivo IL-6, suggesting distinct effects of IL-6 under in vitro and in vivo conditions (Fig. 6E). Bcl-2 levels, however, remained unaffected by both in vitro and in vivo IL-6, which documents a pro-survival pathway of IL-6 that is distinct from common γc-chain cytokines. Altogether, these data propose a role for IL-6 as a pro-survival homeostatic cytokine for naïve T cells in vivo.
4. Discussion
In vivo availability of homeostatic cytokines determines the size of the peripheral T cell pool. In this study, we identified the pro-inflammatory cytokine IL-6 as a novel homeostatic cytokine that expands T cell space by inducing expression of anti-apoptotic genes, such as Mcl-1, and by upregulating expression of pro-metabolic genes, such as Pim-1 and Pim-2. Interestingly, IL-6 overexpression did not promote thymopoiesis or induce T cell activation, but it acted specifically on resting T cells to increase T cell space. Since IL-6 expression is highly upregulated during immune activation and inflammation, IL-6 mediated T cell survival could represent a mechanism to maintain the T cell pool when signaling by other homeostatic cytokines becomes limiting.
Naïve T cell survival is dependent on IL-7 [2, 4, 49]. T cell activation, however, induces rapid downregulation of IL-7 receptor expression so that factors other than IL-7 must mediate T cell survival during immune activation [18, 50]. Previously, a set of cytokines have been identified to promote T cell survival, which includes the γc cytokines IL-2, IL-4, IL-7, IL-15, and the gp130 family cytokine, IL-6 [51, 52]. These cytokines not only prevented apoptosis but also provided trophic effects to maintain cell size and induce cell metabolism. Notably, all these pro-survival cytokines share the common signaling pathways of JAK-STAT and PI3-Kinase/Akt activation [12, 33, 53]. This observation suggests that pro-survival cytokines could be potentially interchangeable and redundant in their effects to provide T cell survival and homeostasis. Whether such is the case, and if so, which cytokine could possibly function redundant to IL-7 has not been clear. However, T cell adoptive transfer experiments into IL-7 deficient mice documented that, at least under resting conditions, IL-7 is a non-redundant factor in T cell survival [2, 4]. Thus, IL-7 is an essential requirement for T cell survival, even in an environment sufficient in other pro-survival cytokines [49]. Our current data now demonstrate that IL-6 can have an additive survival effect to endogenous IL-7, as IL-6 overexpression further expanded peripheral T cell space under IL-7 sufficient conditions. Importantly, IL-6 increased T cell numbers independent of T cell differentiation status, i.e. being naïve or activated/memory phenotype cells. Thus, we identified IL-6 as a bona fide homeostatic cytokine in T cells, with a potential role in providing T cell survival under inflammatory conditions.
A role of pro-inflammatory cytokines in T cell survival has been demonstrated in earlier studies. For example, T cell priming to tolerogenic antigens was greatly enhanced by injection of bacterial lipopolysaccharide (LPS) [54]. In bacterial superantigen-stimulated T cells, pro-inflammatory cytokines such as TNFα or IFNγ protected T cells from activation induced cell death, presumably by converting them into a Fas-resistant state [55]. Specifically, co-injection of LPS inhibited the deleterious effect of Staphylococcal enterotoxin A (SEA) on Vβ3+ CD4 T cells in a TNFα dependent manner [55]. Thus, pro-inflammatory cytokines have been previously appreciated as pro-survival factors during T cell activation. A role for pro-inflammatory IL-6, however, has been less clear. Subsequent studies on SEA stimulated T cells reported that IL-6 failed to prevent cell death in activated cells or in memory phenotype cells (CD44hiCD62Llo) in vitro, and that IL-6 promoted cell survival of bystander resting T cells rather than of activated cells [56]. In contrast to such reports, however, IL-6 was reported to promote survival and expansion of activated T cells in vivo. Specifically, IL-6 improved survival and expansion of primed 5CC7 TCR transgenic CD4 T cells in vivo [57], and rescued naïve CD4 T cells from TCR-induced cell death without Th1/Th2 polarization in vivo [58]. Along these lines, IL-6 also reduced activation induced cell death in T cells independent of IL-2 production [59]. Thus, an IL-6 pro-survival effect in activated T cells remains controversial and possibly reflects a difference of in vivo and in vitro experimental systems.
In addition to its role under activating/inflammatory conditions, a role for IL-6 under steady-state conditions also has remained unclear. While peripheral T cell numbers in thymectomized IL-6 deficient mice did not show an accelerated decrease compared to thymectomized wildtype mice [60], a direct analysis of IL-6 deficient mice reported a 20–40% reduction in total number of thymocytes and peripheral T cells [61]. Whether this is a direct effect on T cells or an indirect effect on dendritic cells and stromal cells is not known [62, 63]. Altogether, an IL-6 effect on T cell maintenance in vivo has been inconclusive.
Assessing long-term homeostatic effects of IL-6 on T cells has been difficult because of its pleiotropic effect on other immune cells, specifically B cells [36, 37]. As such, IL-6 transgenic mice display enlarged peripheral lymphoid organs and are prone to inflammation and autoimmunity [36]. The major pathology of IL-6 transgenic mice is manifested in massive polyclonal plasmacytosis, a dramatic increase in serum IgG1 levels, and renal failure that leads to premature death [36]. In the T cell compartment of these mice, both CD4 and CD8 T cells contain a large population of activated CD62Llo phenotype cells, concomitant to increased IL-17 and IFNγ producing CD4 T cells [31]. Thus, IL-6 overexpression induces activation of both B and T cells in vivo. Strikingly, we found that T cells remained quiescent in B cell deficient IgHKOIL6Tg mice, and that it did not result in aberrant accumulation of activated phenotype cells despite an overall increase in T cell numbers. These results indicate that the immunopathology of transgenic IL-6 was B cell dependent and that IL-6 overexpression did not have any direct stimulatory effects on T cells. Why IL-6 signaled B cells impose a pro-inflammatory environment for T cells is an interesting question. Identification of B cell dependent factors that trigger T cell activations and inflammation in B cell sufficient IL-6Tg mice is currently under investigation.
In addition to upregulation of survival factors, another striking feature of in vivo IL-6 signaling was the downregulation of IL-7Rα expression in trans. Previously, we showed that pro-survival cytokines, including IL-6, suppressed IL-7Rα expression in vitro [18]. Thus, IL-7Rα downregulation by IL-6 in vivo is in agreement with these results. We consider this mechanism important to exclude IL-6 signaled cells from being redundantly signaled by IL-7 so that repetitive pro-survival signaling and unnecessary consumption of IL-7 are prevented. Moreover, IL-7Rα downregulation by IL-6 in trans will also maximize in vivo availability of IL-7 for cells that failed to get signaled by IL-6. Thus, we propose that cross-talk and balancing of IL-6Rα and IL-7Rα expression act synergistically to optimize pro-survival cytokine consumption and maximize T cell homeostasis.
Finally, while we demonstrated that IL-6 can act as a homeostatic T cell survival factor, our data also indicate that its pro-survival mechanism is distinct from that of IL-7. Both IL-6 and IL-7 activates the JAK/STAT signaling pathway. However, STAT5 is the major signaling molecule for IL-7 whereas STAT3, and to a lesser extent STAT1, is the primary downstream molecule of IL-6 [35, 64, 65]. Consistent with such divergent signaling pathways, we found that Bcl-2 mRNA expression was induced by IL-7 but not by IL-6. Mcl-1 expression, on the other hand, was induced by both IL-7 and IL-6, suggesting that IL-7 exerts a broader and more potent anti-apoptotic effect than IL-6. It would be important to assess to what extent IL-6 overexpression could replace IL-7 deficiency during thymopoiesis and T cell homeostasis. Generation of IL-6 transgenic mice on an IL-7 deficient background could answer these questions, and we are currently in the process of generating such mice. Together with in vitro cytokine co-stimulation studies, we expect that these tools will provide insights on the unique role of IL-7 and synergistic effects of IL-6 in T cell survival.
Collectively, here we demonstrated a homeostatic effect of IL-6 in T cell survival that was revealed through the genetic depletion of B cells. This system allowed us to assess a direct effect of IL-6 on T cell survival, and we demonstrate that IL-6 promotes survival of resting T cells and expands peripheral T cell space.
Supplementary Material
Highlights.
IL-6 signaling downregulates expression of its own receptor
IL-6 overexpression increases T cell numbers without affecting thymopoiesis
IL-6 signaling promotes cell survival by inducing expression of Mcl-1 but not Bcl-2
IL-6 is a novel homeostatic cytokine for naïve T cells
Acknowledgments
We thank Drs. A. Singer and R. Etzensperger for critical review of this manuscript. We thank Drs. Wayne Chu and Phil Lucas for providing us reagents and protocol for performing TREC assays. This work was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–6. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 3.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–26. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–41. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 6.Khaled AR, Li WQ, Huang J, Fry TJ, Khaled AS, Mackall CL, et al. Bax deficiency partially corrects interleukin-7 receptor alpha deficiency. Immunity. 2002;17:561–73. doi: 10.1016/s1074-7613(02)00450-8. [DOI] [PubMed] [Google Scholar]
- 7.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–6. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 8.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–11. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallard C, Stegmann AP, van Kleffens T, Smart F, Venkitaraman A, Spits H. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10:525–35. doi: 10.1016/s1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci U S A. 1997;94:3627–32. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benczik M, Gaffen SL. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunological investigations. 2004;33:109–42. doi: 10.1081/imm-120030732. [DOI] [PubMed] [Google Scholar]
- 13.Allard EL, Hardy MP, Leignadier J, Marquis M, Rooney J, Lehoux D, et al. Overexpression of IL-21 promotes massive CD8+ memory T cell accumulation. Eur J Immunol. 2007;37:3069–77. doi: 10.1002/eji.200637017. [DOI] [PubMed] [Google Scholar]
- 14.Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–31. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida Y, Nishi M, Taguchi O, Inaba K, Minato N, Kawaichi M, et al. Effects of the deregulated expression of human interleukin-2 in transgenic mice. Int Immunol. 1989;1:113–20. doi: 10.1093/intimm/1.2.113. [DOI] [PubMed] [Google Scholar]
- 16.Ruger BM, Erb KJ, He Y, Lane JM, Davis PF, Hasan Q. Interleukin-4 transgenic mice develop glomerulosclerosis independent of immunoglobulin deposition. Eur J Immunol. 2000;30:2698–703. doi: 10.1002/1521-4141(200009)30:9<2698::AID-IMMU2698>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Ligons DL, Tuncer C, Linowes BA, Akcay IM, Kurtulus S, Deniz E, et al. CD8 Lineage-specific Regulation of Interleukin-7 Receptor Expression by the Transcriptional Repressor Gfi1. J Biol Chem. 2012;287:34386–99. doi: 10.1074/jbc.M112.378687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Reem GH, Yeh NH, Urdal DL, Kilian PL, Farrar JJ. Induction and upregulation by interleukin 2 of high-affinity interleukin 2 receptors on thymocytes and T cells. Proc Natl Acad Sci U S A. 1985;82:8663–6. doi: 10.1073/pnas.82.24.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renz H, Domenico J, Gelfand EW. IL-4-dependent up-regulation of IL-4 receptor expression in murine T and B cells. J Immunol. 1991;146:3049–55. [PubMed] [Google Scholar]
- 21.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–28. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 23.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–26. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 24.Okuda Y, Sakoda S, Bernard CC, Fujimura H, Saeki Y, Kishimoto T, et al. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10:703–8. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 25.Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol. 1998;28:2178–87. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178::AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–8. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–82. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 28.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 29.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 30.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto M, Nakano M, Terabe F, Kawahata H, Ohkawara T, Han Y, et al. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol. 2011;186:32–40. doi: 10.4049/jimmunol.0903314. [DOI] [PubMed] [Google Scholar]
- 32.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300:2101–4. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 33.Chen RH, Chang MC, Su YH, Tsai YT, Kuo ML. Interleukin-6 inhibits transforming growth factor-beta-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways. J Biol Chem. 1999;274:23013–9. doi: 10.1074/jbc.274.33.23013. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 36.Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989;86:7547–51. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, et al. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1992;89:232–5. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 39.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–54. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tajima M, Wakita D, Noguchi D, Chamoto K, Yue Z, Fugo K, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med. 2008;205:1019–27. doi: 10.1084/jem.20071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawa S, Kamimura D, Jin GH, Morikawa H, Kamon H, Nishihara M, et al. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J Exp Med. 2006;203:1459–70. doi: 10.1084/jem.20052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–70. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 45.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–52. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036–42. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 47.MacDonald HR, Budd RC, Cerottini JC. Pgp-1 (Ly 24) as a marker of murine memory T lymphocytes. Curr Top Microbiol Immunol. 1990;159:97–109. doi: 10.1007/978-3-642-75244-5_6. [DOI] [PubMed] [Google Scholar]
- 48.Swain SL, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, et al. From naive to memory T cells. Immunol Rev. 1996;150:143–67. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim GY, Ligons DL, Hong C, Luckey MA, Keller HR, Tai X, et al. An in vivo IL-7 requirement for peripheral Foxp3+ regulatory T cell homeostasis. J Immunol. 2012;188:5859–66. doi: 10.4049/jimmunol.1102328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue HH, Kovanen PE, Pise-Masison CA, Berg M, Radovich MF, Brady JN, et al. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc Natl Acad Sci U S A. 2002;99:13759–64. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–76. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 52.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. J Immunol. 1997;158:5791–6. [PubMed] [Google Scholar]
- 53.Ramji DP, Vitelli A, Tronche F, Cortese R, Ciliberto G. The two C/EBP isoforms, IL-6DBP/NF-IL6 and C/EBP delta/NF-IL6 beta, are induced by IL-6 to promote acute phase gene transcription via different mechanisms. Nucleic Acids Res. 1993;21:289–94. doi: 10.1093/nar/21.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Louis JA, Chiller JM, Weigle WO. The ability of bacterial lipopolysaccharide to modulate the induction of unresponsiveness to a state of immunity. Cellular parameters. J Exp Med. 1973;138:1481–95. doi: 10.1084/jem.138.6.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261–70. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 56.Teague TK, Schaefer BC, Hildeman D, Bender J, Mitchell T, Kappler JW, et al. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. J Exp Med. 2000;191:915–26. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survival. J Immunol. 2005;174:4761–7. doi: 10.4049/jimmunol.174.8.4761. [DOI] [PubMed] [Google Scholar]
- 58.Ben-Sasson SZ, Makedonski K, Hu-Li J, Paul WE. Survival and cytokine polarization of naive CD4(+) T cells in vitro is largely dependent on exogenous cytokines. Eur J Immunol. 2000;30:1308–17. doi: 10.1002/(SICI)1521-4141(200005)30:5<1308::AID-IMMU1308>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 59.Ayroldi E, Zollo O, Cannarile L, FDA, Grohmann U, Delfino DV, et al. Interleukin-6 (IL-6) prevents activation-induced cell death: IL-2-independent inhibition of Fas/fasL expression and cell death. Blood. 1998;92:4212–9. [PubMed] [Google Scholar]
- 60.Vivien L, Benoist C, Mathis D. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int Immunol. 2001;13:763–8. doi: 10.1093/intimm/13.6.763. [DOI] [PubMed] [Google Scholar]
- 61.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez Mdel C, Bernad A, Aracil M. Interleukin-6 deficiency affects bone marrow stromal precursors, resulting in defective hematopoietic support. Blood. 2004;103:3349–54. doi: 10.1182/blood-2003-10-3438. [DOI] [PubMed] [Google Scholar]
- 63.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–54. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 64.Gerhartz C, Heesel B, Sasse J, Hemmann U, Landgraf C, Schneider-Mergener J, et al. Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. I. Definition of a novel phosphotyrosine motif mediating STAT1 activation. J Biol Chem. 1996;271:12991–8. doi: 10.1074/jbc.271.22.12991. [DOI] [PubMed] [Google Scholar]
- 65.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–9. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.