Figure 3.
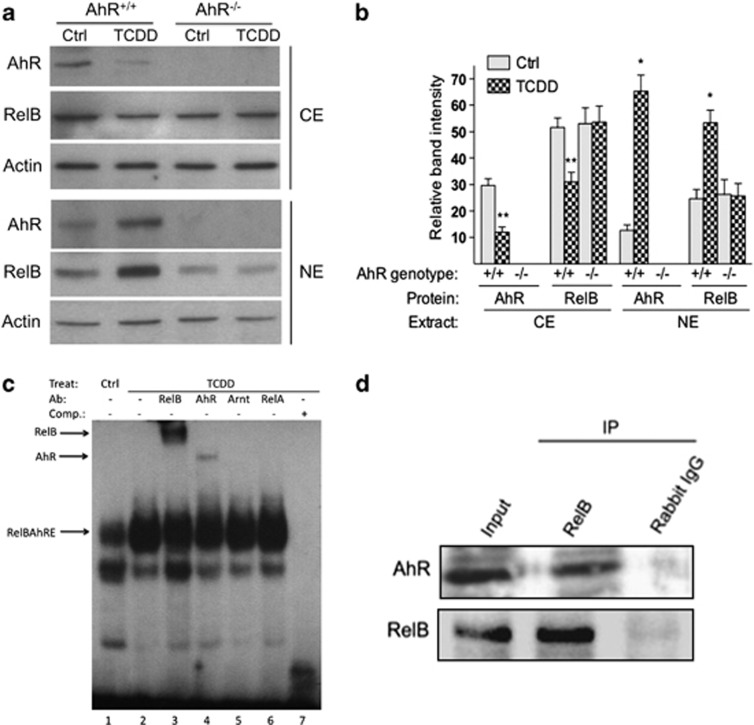
AhR controls TCDD-stimulated nuclear accumulation and DNA-binding activity of RelB in BMDC. (a) Levels of AhR and RelB proteins in cytosolic extract (CE) and nuclear extract (NE) of BMDCs from B6 wt (AhR+/+) or AhR-null mice (AhR−/−) are shown. Bone marrow cells were cultured for 7 days in RPMI with GM-CSF in presence or absence of 1 nM TCDD. AhR, RelB and Actin proteins were analyzed using the western blot analysis. One representative experiment out of three is presented. (b) Densitometric evaluation of band intensities of the AhR and RelB protein bands. Results of three independent experiments are shown as mean values±s.d. (*, significantly higher than control P<0.05; **, significantly lower than control P<0.05). (c) TCDD-enhanced binding of AhR and RelB to a RelBAhRE-binding element during DC differentiation. Electromobility-shift assay (EMSA) was performed with nuclear extracts derived from BMDCs incubated with32P-end-labeled double-stranded oligonucleotide coding for AhR/RelB binding as described earlier.10 Lanes 3 and 4 show bandshift reaction mixtures that were incubated additionally with antibodies against AhR and the NF-κB family member RelB. Addition of antibodies against Arnt (lane 5) and RelA (lane 6) did not cause a supershift or decrease in binding activity. A 200-M excess of wild-type cold-competition oligonucleotide (lane 5) has also been included as control. An arrow indicates a binding reaction (supershift and/or decrease in bandshift intensity). Ab, Antibody; Comp, competition; Ctrl, control; Treat, treatment. One representative experiment out of three independently performed experiments is shown. (d) Detection of the AhR/RelB complex in nuclear extracts of BMDC. Bone marrow cells were cultured in RPMI containing 20 ng ml−1 GM-CSF for 7 days. RelB and AhR immunoprecipitates were prepared with nuclear extracts collected from wt BMDC. The antigen–antibody complexes were visualized via chemoluminescence. Polyclonal NF-κB member RelB (Active Motif, Carlsbad, CA, USA) and polyclonal AhR (Novus Biologicals, Littleton, CO, USA) antibodies were used for western blot, Supershift in EMSA and co-immunoprecipitation. Data are representative of three independent experiments.