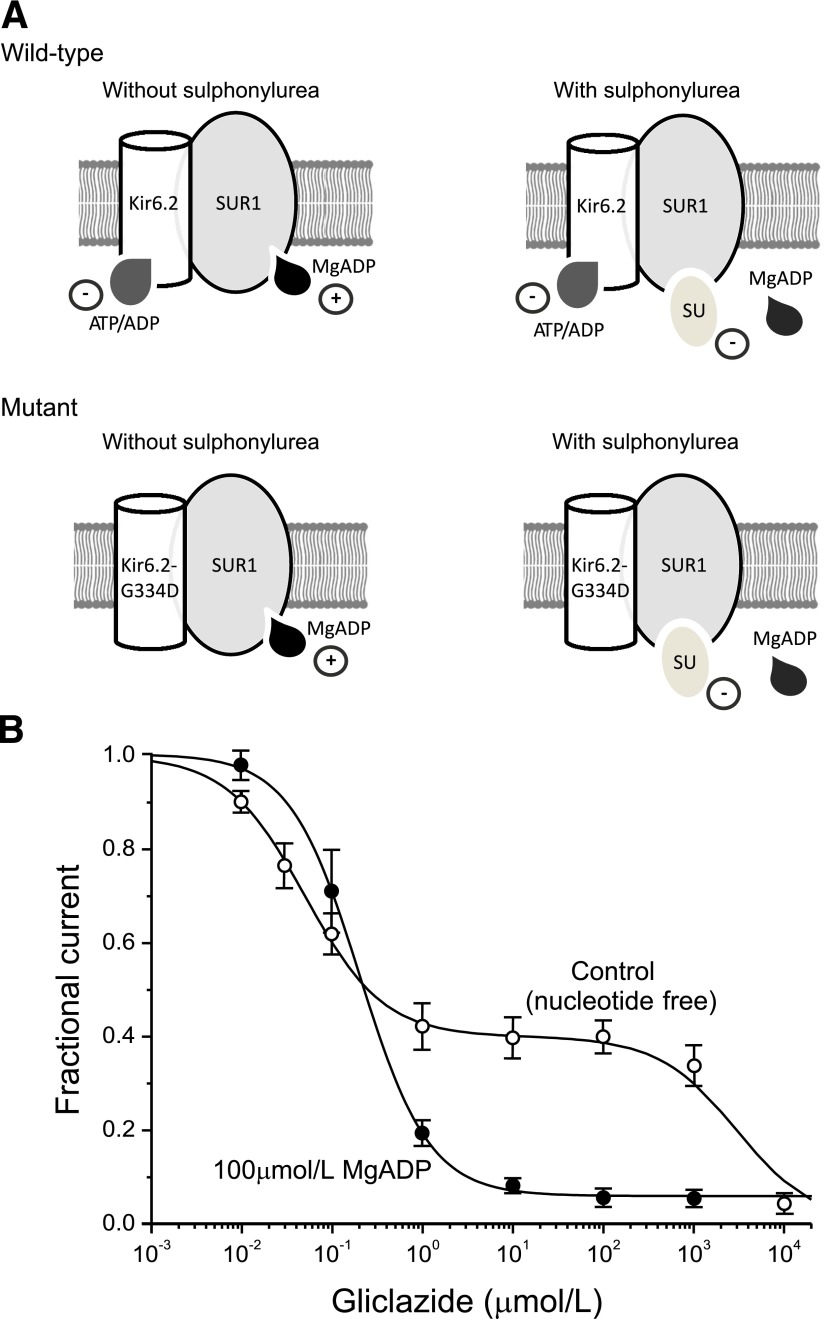
FIG. 1.
The mechanism of sulphonylurea block of KATP channels. A: Schematic of the interaction of Mg-nucleotides with Kir6.2 and SUR1 subunits in the absence (left) and presence (right) of sulphonylureas for wild-type channels and channels carrying an ATP-insensitive mutation (G334) in Kir6.2. Nucleotides reduce the PO of wild-type channels by binding to Kir6.2 and increase PO by interacting with SUR1 (top left). Sulphonylureas inhibit channel activity by binding to SUR1. They also displace MgADP from SUR1, and consequently, ATP block at Kir6.2 is not balanced by MgATP activation at SUR1 (top right). Channels with a mutation that abolishes (G334D) ATP inhibition at Kir6.2 are activated but not blocked by ADP or MgADP (bottom left). Sulphonylureas still inhibit channel activity. They also displace MgADP from SUR1, abolishing activation; however, the total block is less because of the lack of ATP inhibition at Kir6.2 (bottom right). B: Concentration-response curves for gliclazide block of macroscopic KATP currents in excised patches in the absence (○; n = 4–8) and presence (●; n = 7–12) of 100 μmol/L MgADP. The lines are the best fit of Eq. 2 to the mean data, with IC50(1) = 50 nmol/L, IC50(2) = 3 mmol/L, h1 = h2 = 1, and a = 0.4 (0 µmol/L MgADP), or to Eq. 1, with IC50 = 204 nmol/L, h = 1.13, and a = 0.065 (100 µmol/L MgADP). Data are taken from Gribble and Ashcroft (23) and Proks et al. (33). SU, sulphonylurea.