Summary
There is a strong relationship between socioeconomic status (SES) and health outcomes in the U.S, though the mechanisms are poorly understood. Increasing evidence points to links between lifelong exposure to infectious disease and subsequent chronic disease. Exposure and susceptibility to infections may be one way SES affects long-term health, though little population-based research to date has examined social patterning of infections in the U.S. This paper tests the relationship between income, education, race/ethnicity and seroprevalence of cytomegalovirus (CMV) infection at different ages in a representative sample of the U.S. population, and tests potential mediators for these relationships. The study finds significant racial and socioeconomic disparities in CMV seroprevalence beginning at early ages and persisting into middle age. Potential exposures do not explain the relationship between socioeconomic status and CMV positivity. Because reactivation of latent CMV infections may contribute to chronic disease and immune decline later in life, future research should determine the exposure or susceptibility pathways responsible for these disparities in the prevalence of CMV infection.
Keywords: Socioeconomic factors, Immunity, Latent Infection, Cytomegalovirus
Introduction
Disparities in morbidity and mortality by socioeconomic status (SES) and race/ethnicity in the U.S. are well established (1, 2). The U.S. Department of Health and Human Services declared as one of the two major goals of its Healthy People 2010 health promotion agenda "to eliminate health disparities among different segments of the population."(3) Despite this public concern, the physical mechanisms underlying health disparities remain poorly understood. Likely candidates such as health behaviours and access to health care have not easily accounted for the gradient (1, 4). Increasing evidence points to links between lifelong exposure to infectious disease and subsequent chronic disease, suggesting a potential mechanism for linking SES to health outcomes (5–7). Low social status has been linked to increased risk of respiratory infections in humans and other primates in experimental studies (8–10). Much less is known about the links between social status and susceptibility to infections in the broader U.S. population.
Exposure to herpesviruses such as cytomegalovirus (CMV) is nearly ubiquitous in early life, and is even found in isolated aboriginal groups (11, 12). Primary infection during pregnancy is a leading cause of hearing loss, vision loss, and mental retardation among congenitally infected children (13). Although infection with CMV often passes undiagnosed because of its asymptomatic properties, the virus remains persistent in the host’s cells for life. Adequate cell-mediated immunity is important for maintaining the virus in this chronic state (14, 15). Importantly, CMV has been linked to inflammatory processes, cardiovascular disease, cognitive outcomes, and Alzheimer’s disease (11, 16–20). For these reasons, it is important to examine the prevalence of CMV at various life stages within diverse socioeconomic and racial groups.
Racial/ethnic differences in infection status for CMV have been described in the U.S. adjusting for socioeconomic status (12, 13). Age-adjusted seroprevalence rates for CMV were found to be 81.7% for Mexican Americans, 75.8% for non-Hispanic Blacks, and 51.2% for non-Hispanic Whites (12). An age-adjusted association between three categories of family income and CMV seroprevalence was found in the U.S., with this relationship diminishing in a multivariate model adjusting for age, race/ethnicity, education, marital status, area of residence, census region, family size, country of birth, and type of medical insurance (12). These studies did not explicitly examine the relationship between education, income and prevalence of the infection in different age groups or test pathways that might explain SES differences in infection status. This paper will examine differences in CMV seropositivity by education, income, and race/ethnicity at different ages, then test whether variables proxying for potential exposure can explain the relationship between SES, race/ethnicity, and infection status.
Although previous research has demonstrated overall socioeconomic and racial/ethnic disparities in seropositivity, it is unclear at what age social gradients in infection emerge and what factors might explain these gradients. There are no studies of which we are aware that have examined education and income gradients in CMV infection status across age in a nationally representative sample from the U.S. population and tested the role of potential exposure pathways that might explain these differentials. These are important questions, since the later life consequences of CMV infection for cell-mediated immunity may depend on the lifetime burden of this infection, and understanding how socioeconomic and racial/ethnic groups are differentially exposed and/or susceptible to this infection can help us design effective interventions. This paper will examine the relationship between socioeconomic status, as measured by household income and years of education, and race/ethnicity, and the presence of serum IgG antibodies to CMV in persons ages 6 and older in the U.S. Looking separately at children and adults, we will test how well these disparities can be accounted for by mediating variables that have been posited as potential exposure risk factors such as the number of individuals in the household, nativity outside of the U.S., region of residence, day care attendance for youth, and sexual behaviors for adults.
Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) III was conducted by the National Center for Health Statistics from 1988 to 1994 and was a complex, stratified, multistage probability cluster sample of the US population including interview, clinical examination, and laboratory tests. Persons <5 or >60 years of age, non-Hispanic black persons, and Mexican Americans were sampled at a higher frequency than other groups to obtain adequate sample sizes to more accurately evaluate these subgroups. The complete methodology and response rates of NHANES III have been published previously(21). Surplus sera data containing positive or negative measures for CMV IgG antibody were released in 2005 for respondents aged 6 and over, thus these data represent the most recent data available on CMV prevalence in the U.S. population. Our analysis focuses on two age groups. For ages 6–16, 4565/6936 of interviewed respondents also had information on CMV serostatus and were included in our analysis. Those missing CMV data were more likely to be non-Hispanic white, with slightly higher family income and education of the household reference person. Another 416 observations in this age group are dropped due to missing information on one or more covariates. For ages 25–49, of the 8230 interview respondents, 7255 had information on CMV serostatus. Those missing CMV data were not significantly different with respect to age, sex, race, income, or education. Another 1219 observations were dropped in this age category due to missing values on one or more covariates. Compared to the non-missing observations, those dropped were more likely to be non-Hispanic black, female, and have less education. All analyses were conducted using STATA 10.0 survey commands to account for the complex survey design, and the sample completing the laboratory components was weighted to reflect the non-institutionalized population of the United States.
Laboratory Analyses
Serum samples were tested for CMV specific IgG with a Triturus robot (Grifols USA) with SeraQuest enzyme-linked immunoabsorbent assay (ELISA) reagents by Quest International, Inc., Miami FL. Reference ranges of positive and negative controls, and standards were established by the MLE (Master Lot Entry) card for each lot. ELISA Optical Density Index values greater than 1.0 were considered positive.
Sera with values near the ELISA cut-off, between .90 and 1.05 (approx 7% of total), were confirmed with a second ELISA assay by bioMerieux, Inc., Durham, NC. When the results from these 2 tests disagreed (approx. 2% of total), an IFA from Bion International, Inc., was used and the result of the IFA was given as the final result. This procedure resulted in 98% sensitivity and 99% specificity(12, 22).
Statistical Analyses
Logistic regression models were used to test the relationship between CMV positivity status (0=negative, 1=positive), race/ethnicity (non-Hispanic White, Non-Hispanic Black, Mexican-American, and Other), years of education, and family income. For those under 17, education is measured as the highest grade completed by the household reference person. For those 25 and over, education is measured as the respondent’s years of completed education. Family income is coded as the midpoint of each of the 26 reported categories (using $65,000 for the incomes above $50,000), adjusted for inflation between the two NHANES III phases using the Consumer Price Index. Income was then log-transformed due to the skewness of the distribution. Variables potentially mediating exposure included in all models were age at the time of exam, housing crowdedness (persons in dwelling/number of rooms), an indicator for urban or rural residence, census region of residence, and gender. Results using alternative specifications of family and household size, including total number of persons, total number of children, and log (household size) did not significantly differ from our measure of housing crowdedness. For respondents under age 17, controls include an indicator for daycare attendance before the age of 4, an indicator for whether the child was born outside the U.S., and an indicator for whether the child’s mother was born outside the U.S. For respondents aged 25–49, age at first sexual intercourse, an indicator for more than 10 total lifetime sexual partners, and whether the respondent was born outside of the U.S. are included as potential exposure mediators. Preliminary models included continuous measures of number of lifetime sexual partners, but the dichotomous measure provided the best fit. To account for potential increased susceptibility to infections, an indicator for whether the child was born low birth weight (< 2500 grams) and whether there is a smoker in the home was included for respondents under 17, and an indicator for current, former or never smoking was included for adult respondents.
Sexual history questions were not asked of adults over age 49, and thus these adults are included in the basic analysis represented in Figure 1 but not included in models testing potential mediators. Due to concern over measurement of income and education for college age individuals, respondents aged 18–24 are also included in basic analysis for Figure 1 but excluded from the full models testing potential mediators of the relationship between income, education, and CMV seropositivity.
Figure 1.
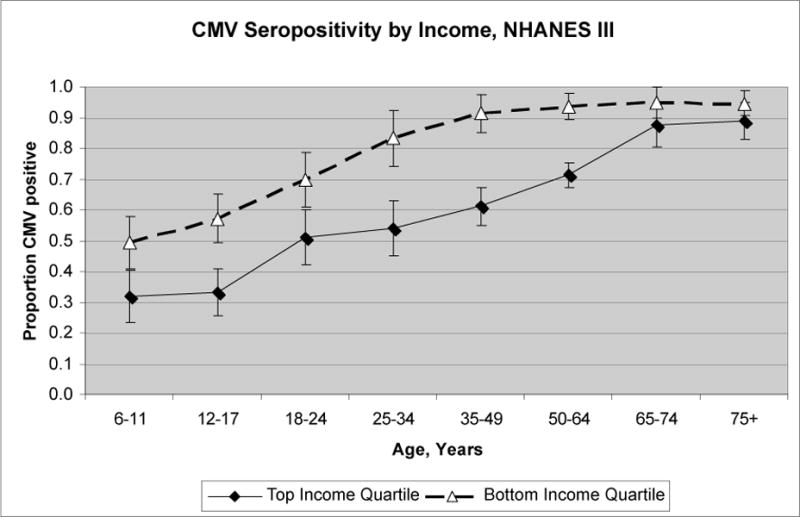
Data from the Third National Health and Nutrition Examination Survey, 1988–1994, showing seroprevalence of cytomegalovirus (CMV) by age and income quartile. Results were adjusted for race/ethnicity, rural/urban residence, census region, sex, and household size.
Results
Figure 1 depicts rates of CMV seropositivity by age for the highest and lowest income quartile in NHANES III, adjusted for race/ethnicity, rural/urban residence, census region, sex, and household size. We see that cross-sectionally, income differences in seropositivity appear at early ages, widen in early and middle adulthood, then converge at older ages as almost everyone in the population is exposed. Table 1 shows descriptive statistics for our study sample. Table 2 shows the odds ratios for CMV seropositivity for ages 6–11 and 12–16. The first model estimates the effect of race/ethnicity and household socioeconomic status on the odds of CMV seropositivity for children ages 6–11 without adjustment for potential mediators. Adjusting for household socioeconomic status, non-Hispanic black children aged 6–11 have 37% higher odds of being infected with CMV compared to non-Hispanic whites, though the confidence interval includes the null value. Mexican-American children have 93% higher odds of being infected in the age 6–11 group compared to non-Hispanic whites. Both education of the reference person and family income are significantly related to infection status controlling for race/ethnicity, with each additional year of education of the family reference person corresponding to a 5% decrease in the odds of infection. Moving from a family income of $15000 a year to $40000 is associated with 14% lower odds of infection. Next we add controls for markers of potential exposures and susceptibility. Living in the northeast is associated with lower odds of infection, while household crowding and nativity of the mother outside of the U.S. are significantly related to greater odds of infection for the 6–11 age group. Living in a rural versus an urban area, daycare attendance, having a smoker in the household, and being born low birth weight are not significantly associated with infection status controlling for other factors. The addition of these potential mediators eliminates the racial/ethnic and parental educational differences in infection status in children aged 6–11, while the differences in CMV seropositivity by income remain unchanged. For children aged 12–16, the results are similar. Racial/ethnic differences in seropositivity are eliminated upon inclusion of mediators, but for older children the differences by both parental education and family income remain.
Table 1.
Sample Descriptive Statistics(Weighted) NHANES III, 1988–1994
| Percentage or Mean and Linearized Standard Error
| ||
|---|---|---|
| Ages 6–16 | Ages 25–49 | |
| CMV Positive | 38.6% | 57.1% |
| Non-Hispanic White | 67.7% | 76.9% |
| Black | 14.5% | 11.3% |
| Mexican-American | 13.4% | 5.03% |
| Male | 51.3% | 49.1 |
| Years of Education† | 12.5 (.11) | 13 (.08) |
| Annual Family Income | 36864.5 (1145.7) | 41432 (809) |
| Housing Density (Persons/Rooms) | 0.79 (0.01) | 0.61 (.01) |
| Before age 4, attend day care w/ 6+ kids | 46% | N/A |
| Smoker in Household | 39.1% | N/A |
| Child was born low birthweight | 2.7% | N/A |
| Mother born outside U.S. | 15.6% | N/A |
| Born outside the U.S | 0.07 | 11.5% |
| Never Smoker | N/A | 45% |
| Former Smoker | N/A | 21.3% |
| Current Smoker | N/A | 33.7% |
| >10 Lifetime Sexual Partners | N/A | 32.4% |
| Age at First Intercourse | N/A | 17.6 (.08) |
| N= | 4149 | 6036 |
Highest grade completed by household reference person for ages 6–16
Table 2.
Risk factors for CMV seropositivity under age 17, National Health and Nutrition Examination Survey, 1988–1994.
|
|
Age 6–11 (n=2434) OR [95% CI] |
Age 12–16 (n=1786) OR [95% CI] |
||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Non-Hispanic White | 1.0 | 1.0 | 1.0 | 1.0 |
| Non-Hispanic Black | 1.37 (0.99–1.90]) | 1.28 (0.93–1.76) | 1.27 (0.91–1.77) | 1.16 (0.83–1.63) |
| Mexican-American | 1.93 (1.41–2.63) | 1.14 (0.80–1.65) | 1.75 (1.23–2.49) | 1.16 (0.76–1.75) |
| Education of family reference person | 0.95 (0.92–0.98) | 0.99 (0.95–1.02) | 0.91 (0.88–0.94) | 0.93 (0.90–0.97) |
| Log(Family Income) | 0.86 (0.77–0.96) | 0.83 (0.73–0.94) | 0.85 (0.77–0.95) | 0.86 (0.76–0.96) |
| Male | 1.01 (0.86–1.17) | 0.67 (0.56–0.80) | ||
| USDA code-Rural | 1.08 (0.87–1.33) | 1.09 (0.84–1.41) | ||
| Census Region- Northeast | 1.0 | 1.0 | ||
| Census Region-Midwest | 1.70 (1.09–2.66) | 1.01 (0.65–1.56) | ||
| Census Region-South | 1.88 (1.27–2.77) | 1.50 (1.11–2.04) | ||
| Census Region—West | 1.91 (1.25–2.93) | 1.43 (0.99–2.05) | ||
| Housing Density (Persons/Rooms) | 1.31 (1.03–1.66) | 1.34 (1.02–1.77) | ||
| Before age 4, attend day care w/ 6+ kids | 1.05 (0.85–1.31) | 1.17 (0.94–1.46) | ||
| Smoker in the Household | 0.83 (0.68–1.02) | 1.17 (0.97–1.40) | ||
| Child was born low birthweight | 1.29 (0.96–1.74) | 0.65 (0.30–1.44) | ||
| Born outside U.S. | 1.44 (0.88–2.34) | 2.29 (1.56–3.37) | ||
| Mother born outside U.S. | 2.40 (1.75–3.31) | 1.79 (1.29–2.49) | ||
all models also control for age in months
Table 3 presents results for adults aged 25–49 separately by sex. Relative differences in seropositivity by race/ethnicity in this age range have greatly increased compared to the childhood sample, with non-Hispanic black men having almost 4 times the odds of infection compared to non-Hispanic white men, and Mexican-American men having almost 5 time the odds of infection. The differences are even larger for women. Differences in infection status by education and income are also larger than the gaps at younger ages. Including controls for potential exposure, we see that being born outside of the U.S. is a large, positive predictor of infection status in adults. Living in the south is also associated with higher odds of infection for both men and women. Population-based studies have suggested that sexual transmission is a likely risk factor for exposure to CMV (12, 23). Age at first intercourse and having more than ten lifetimes sexual partners are significant predictors of CMV infection status only for women. Household size and rural versus urban residence are not associated with seropositivity in adults. Smoking is associated with a decrease in some immune markers, and thus may increase susceptibility to infection (24). Interestingly, being a current smoker is significantly associated with infection status for both men and women, but in opposite directions. In fully adjusted models, smoking raises the odds of seropositivity for women, while being a current smoker is associated with a lower risk of seropositivity for men. With very little literature on the correlates of CMV infection in the general population, it is difficult to speculate as to the reasons behind these different results for smoking by gender. In models adjusted only for age, smoking remains positively associated with CMV infection for women but is not associated with CMV infection for men. Social factors related to smoking behavior may differ by gender. If this is true, then even controlling for social factors associated with smoking such as race, income, and education, there may be residual unmeasured social factors associated with smoking that are also associated with the likelihood of CMV infection, and these factors may differ by gender. While racial/ethnic differences in positivity diminish slightly upon inclusion of controls for foreign born status, region of residence, and sexual behaviours, they remain large. Importantly, income and education differentials were not explained by exposures commonly cited as risk factors for CMV.
Table 3.
Risk factors for CMV seropositivity ages 25–49, National Health and Nutrition Examination Survey, 1988–1994.
| Men 25–49 (n=2710) OR [95% CI] |
Women 25–49 (n=3326) OR [95% CI] |
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Non-Hispanic White | 1.0 | 1.0 | 1.0 | 1.0 |
| Black | 3.88 (3.06–4.91) | 2.72 (1.98–3.73) | 5.81 (4.49–7.52) | 4.73 (3.51–6.38) |
| Mexican-Americans | 4.73 (3.60–6.20) | 2.27 (1.64–3.15) | 5.67 (3.86–8.32) | 3.93 (2.48–6.20) |
| Years of Completed Education | 0.90 (0.85–0.95) | 0.90 (0.84–0.96) | 0.88 (0.84–0.93) | 0.93 (0.89–0.98) |
| Log(Family Income) | 0.73 (0.59–0.91) | 0.67 (0.53–0.84) | 0.79 (0.66–0.93) | 0.83 (0.70–0.99) |
| USDA code-Rural | 0.91 (0.69–1.18) | 0.88 (0.66–1.16) | ||
| Census Region- Northeast | 1.0 | 1.0 | ||
| Census Region-Midwest | 1.12 (0.69–1.82) | 1.32 (0.96–1.81) | ||
| Census Region-South | 2.0 (1.48–2.70) | 2.32 (1.55–3.49) | ||
| Census Region—West | 1.07 (0.70–1.62) | 1.16 (0.74–1.83) | ||
| Housing Density (Persons/Rooms) | 1.40 (0.96–2.05) | 1.62 (0.99–2.64) | ||
| Former Smoker | 0.77 (0.52–1.12) | 1.15 (0.79–1.67) | ||
| Current Smoker | 0.64 (0.46–0.91) | 1.51 (1.06–2.14) | ||
| Born outside the U.S. | 6.40 (3.48–11.78) | 8.04 (3.66–17.66) | ||
| >10 Lifetime Sexual Partners | 1.33 (0.91–1.93) | 1.62 (1.19–2.20) | ||
| Age at first sexual intercourse – years | 0.98 (0.94–1.02) | 0.93 (0.88–0.98) | ||
all models also adjusted for age in years
Discussion
This study represents a potential new direction for researchers investigating the biophysical mechanisms connecting socioeconomic status and health outcomes. Numerous studies have documented an inverse association between socioeconomic status and several chronic inflammation-associated conditions such as cardiovascular disease and dementia (25–28). The biological pathways that mediate these relationships have not been thoroughly characterized. The social gradient in cardiovascular disease persists even after adjustment for health behaviors and many clinical indicators, suggesting that other processes may be involved (4, 29) It is possible that latent infections such as CMV may explain some of the socioeconomic gradients in cardiovascular disease and possibly other inflammatory-associated conditions. Recent research findings have shown that latent CMV infection in the elderly is an important component of a set of immunological parameters designated the "immunological risk phenotype" (30) This set of immunological markers, including latent CMV infection, high CD8 cells, low CD4 cell percentages, poor T-cell proliferation, is predictive of mortality among healthy elderly individuals.(31–33). While a definitive inference can not be made based on cross-sectional data, the results of this study suggest that individuals with less education, lower income, and non-white race may spend a larger number of years infected with CMV on average by the time they reach older ages. Since the price paid for constant CMV immune vigilance may be high (32), early life disparities in CMV infection could have important implications for disparities in adult health.
Several studies have used herpesviruses, including CMV, as a marker of immune response to psychosocial stress exposures (34). Specifically, increases in herpesvirus antibody titers have been linked to academic stress in medical students and military cadets, (35) caregiving for a family member with Alzheimer’s disease, (36) involvement in a poor quality marriage, (37) anticipation of space flight by astronauts, (38) traumatic life events, (39) as well as psychological traits of loneliness and anxiety (40). It is less clear, however, what role stress may play in initial susceptibility to CMV. Data examining respiratory infections have reported a relationship between low SES, increased levels of stress, and enhanced susceptibility to several cold and flu viruses (9, 10, 41). Low social class was associated with lower secretory immunoglobulin (sIgA), cited as a first line of defense against infection, in a large community sample in Scotland (24). Taken together, these studies suggest that psychological stress associated with low SES can down-regulate various aspects of the cellular immune response, increasing susceptibility to infection. A recent study reported an association between levels of antibody to CMV and level of education in a sample of elderly Latinos (42). While measures of psychosocial stress are not available in the NHANES III data, further studies are needed to assess whether stress mediates the relationship between socioeconomic and race/ethnic differences in CMV susceptibility and antibody response.
There are several limitations to the current study. First, while our analysis has controlled for several exposure variables available in the data such as housing crowdedness, daycare attendance, and number of sexual partners, these variables are likely measured with error. For example, underreporting of the number of sexual partners is probable due to the sensitive nature of the question. Housing crowdedness and family size are imperfect measures of the transmission dynamics that actually determine an individual’s risk of exposure. Being HIV positive or being otherwise immuno-compromised would increase one’s susceptibility to CMV infection, but this information is not available in our data. It is unlikely that these factors explain the differences in seropositivity status seen at early ages. Nonetheless, it is difficult to determine the importance of unmeasured exposures versus susceptibility characteristics in explaining the socioeconomic and racial/ethnic differences in CMV seropositivity. Since the NHANES III data are cross-sectional, we are also not capturing new infections as we look across age groups, but are rather trying to explain differences in prevalence of this infection that could have been acquired at any age prior to measurement. Potential explanatory variables such as sexual behaviors are unlikely to explain CMV infections that were acquired in childhood. Since the seroprevalence rate for the overall 6–11 age group is already 36.3%, there are important predictors of group differences in infection status that are potentially found in unmeasured early life exposures, especially parental or sibling risk factors. Another limitation is that while these are the most recent data available on CMV prevalence in the U.S. population, this sample consists of respondents surveyed from 1988–1994, and thus might be less representative of the current social patterning of CMV infection in the U.S.
In conclusion, we find socioeconomic and racial/ethnic disparities in CMV infection in the U.S. population that are evident as early as age six and persist through middle age. These differentials were not easily explained by exposures commonly cited as risk factors for CMV. Differences in lifelong exposure to persistent infections such as CMV may be one way in which lower social status contributes to poorer health outcomes. Future research should examine the exposure or susceptibility pathways responsible for these disparities in the prevalence of CMV infection.
References
- 1.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: No easy solution. Journal of the American Medical Association. 1993;269(24):3140–5. [PubMed] [Google Scholar]
- 2.Rogers RG, Hummer RA, Nam CB, Peters K. Demographic, Socioeconomic, and Behavioral Factors Affecting Ethnic Mortality by Cause. Social Forces. 1996;74(4):1419–38. [Google Scholar]
- 3.U.S. Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2. Washington, DC: U.S Government Printing Office; Nov, 2000. [Google Scholar]
- 4.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic Factors, Health Behaviors, and Mortality: Results From a Nationally Representative Prospective Study of US Adults. JAMA. 1998;279(21):1703–8. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 5.Nieto FJ. Infections and atherosclerosis: New clues from an old hypothesis? American Journal of Epidemiology. 1998;148(10):937–48. doi: 10.1093/oxfordjournals.aje.a009570. [DOI] [PubMed] [Google Scholar]
- 6.Lindsberg PJ, Grau AJ. Inflammation and Infections as Risk Factors for Ischemic Stroke. Stroke. 2003;34(10):2518–32. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 7.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53(5):747–54. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR. Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosom Med. 1997;59(3):213–21. doi: 10.1097/00006842-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S. Social Status and Susceptibility to Respiratory Infections. Ann NY Acad Sci. 1999;896(1):246–53. doi: 10.1111/j.1749-6632.1999.tb08119.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood Socioeconomic Status and Host Resistance to Infectious Illness in Adulthood. Psychosom Med. 2004;66(4):553–8. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- 11.Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? Journal of Internal Medicine. 2006;259(3):219–46. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 12.Staras SAS, Dollard SC, Radford KW, Dana Flanders W, Pass RF, Cannon MJ. Seroprevalence of Cytomegalovirus Infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–51. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 13.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7(1):71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong MD, Galasso GJ, Gazzard B, et al. Summary of the II International Symposium on Cytomegalovirus. Antiviral Res. 1998;39(3):141–62. doi: 10.1016/s0166-3542(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 15.Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. Journal of General Virology. 2006;87(7):1763–79. doi: 10.1099/vir.0.81891-0. [DOI] [PubMed] [Google Scholar]
- 16.Liu R, Moroi M, Yamamoto M, et al. Presence and severity of chlamydia pneumoniae and cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. International Heart Journal. 2006;47(4):511–9. doi: 10.1536/ihj.47.511. [DOI] [PubMed] [Google Scholar]
- 17.Shen YH, Utama B, Wang J, et al. Human Cytomegalovirus Causes Endothelial Injury Through the Ataxia Telangiectasia Mutant and p53 DNA Damage Signaling Pathways. Circ Res. 2004;94(10):1310–7. doi: 10.1161/01.RES.0000129180.13992.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorlie PD, Nieto FJ, Adam E, Folsom AR, Shahar E, Massing M. A Prospective Study of Cytomegalovirus, Herpes Simplex Virus 1, and Coronary Heart Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2000;160(13):2027–32. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- 19.Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiology of Aging. 2004;25(5):619–27. doi: 10.1016/j.neurobiolaging.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Aiello AE, Haan MN, Blythe L, Moore K, Gonzalez JM, Jagust W. The Influence of Latent Viral Infection on Rate of Cognitive Decline over 4 Years. Journal of the American Geriatrics Society. 2006;54(7):1046–54. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 21.Services USDoHaH, editor. National Center for Health Statistics: Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. 1994. [Google Scholar]
- 22.Antibody to Cytomegalovirus IgG and IgM. NCHS NHANES III Documentation, Codebook Frequencies page. 2006. [Google Scholar]
- 23.Schillinger JA, Xu F, Sternberg MR, et al. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976–1994. Sex Transm Dis. 2004;31(12):753–60. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- 24.Evans P, Der G, Ford G, Hucklebridge F, Hunt K, Lambert S. Social Class, Sex, and Age Differences in Mucosal Immunity in a Large Community Sample. Brain, Behavior, and Immunity. 2000;14(1):41–8. doi: 10.1006/brbi.1999.0571. [DOI] [PubMed] [Google Scholar]
- 25.Diez Roux AV. Persistent social patterning of cardiovascular risk: rethinking the familiar. Circulation. 2005;111(23):3020–1. doi: 10.1161/CIRCULATIONAHA.105.542845. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30(2):256–63. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- 27.Ranjit N, Diez-Roux AV, Chambless L, Jacobs DR, Jr, Nieto FJ, Szklo M. Socioeconomic Differences in Progression of Carotid Intima-Media Thickness in the Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2005 doi: 10.1161/01.ATV.0000198245.16342.3d. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25(1):8–14. doi: 10.1159/000085307. [DOI] [PubMed] [Google Scholar]
- 29.Lynch JWKG, Cohen RD, Tuomilehto J, Salonen JT. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality, and acute myocardial infarction? Am J Epidemiol. 1996;144(10):934–42. doi: 10.1093/oxfordjournals.aje.a008863. [DOI] [PubMed] [Google Scholar]
- 30.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25(8):406–10. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson BO, Ernerudh J, Johansson B, et al. Morbidity does not influence the T-cell immune risk phenotype in the elderly: findings in the Swedish NONA Immune Study using sample selection protocols. Mech Ageing Dev. 2003;124(4):469–76. doi: 10.1016/s0047-6374(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 32.Koch S, Solana R, Rosa OD, Pawelec G. Human cytomegalovirus infection and T cell immunosenescence: A mini review. Mechanisms of Ageing and Development. 2006;127(6):538–43. doi: 10.1016/j.mad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Pawelec G, Koch S, Franceschi C, Wikby A. Human Immunosenescence: Does It Have an Infectious Component? Ann NY Acad Sci. 2006;1067(1):56–65. doi: 10.1196/annals.1354.009. [DOI] [PubMed] [Google Scholar]
- 34.Glaser R. Stress-associated immune dysregulation and its importance for human health: a personal history of psychoneuroimmunology. Brain Behav Immun. 2005;19(1):3–11. doi: 10.1016/j.bbi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Glaser R, Friedman SB, Smyth J, et al. The Differential Impact of Training Stress and Final Examination Stress on Herpesvirus Latency at the United States Military Academy at West Point. Brain, Behavior, and Immunity. 1999;13(3):240–51. doi: 10.1006/brbi.1999.0566. [DOI] [PubMed] [Google Scholar]
- 36.Glaser R, Kiecolt-Glaser JK. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus type 1. Ann Behav Med. 1997;19(2):78–82. doi: 10.1007/BF02883323. [DOI] [PubMed] [Google Scholar]
- 37.Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med. 1993;55(4):364–79. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000;182(6):1761–4. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- 39.McDade TW, Stallings JF, Angold A, et al. Epstein-Barr Virus Antibodies in Whole Blood Spots: A Minimally Invasive Method for Assessing an Aspect of Cell-Mediated Immunity. Psychosom Med. 2000;62(4):560–8. doi: 10.1097/00006842-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Esterling BA, Antoni MH, Kumar M, Schneiderman N. Defensiveness, trait anxiety, and Epstein-Barr viral capsid antigen antibody titers in healthy college students. Health Psychol. 1993;12(2):132–9. doi: 10.1037//0278-6133.12.2.132. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S. The Pittsburgh common cold studies: Psychosocial predictors of susceptibility to respiratory infectious illness. International Journal of Behavioral Medicine. 2005;12(3):123–31. doi: 10.1207/s15327558ijbm1203_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dowd JB, Haan MN, Blythe L, Moore K, Aiello AE. Socioeconomic Gradients in Immune Response to Latent Infection. Am J Epidemiol. 2008;167(1):112–20. doi: 10.1093/aje/kwm247. [DOI] [PubMed] [Google Scholar]


