Table 1.
Selective N7-acylation of 1.a

| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | acylating reagent | product | R | yield | |
| 1 | (CH3CO)2O | 3a | 2a | Me | 78 |
| 2 | (CH3CH2CO)2O | 3b | 2b | Et | 78 |
| 3 | (CH3CH2CH2CO)2O | 3c | 2c | Pr | 73 |
| 4 | [(CH3)2CHCO]2O | 3d | 2d | iPr | 78 |
| 5 | TBSO(CH2)4COOSu | 3e | 2e | TBSO(CH2)4 | 68 |
| 6 |
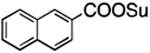
|
3f | 2f |
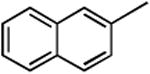
|
91 |
Compound 1 was treated with NaH (1.1 equiv) in DMF for 1 h, then an acylating reagent (1.1 equiv) was added. The yields refer to isolated yields.
