Table 3.
Synthesis of N3-acylated 7.
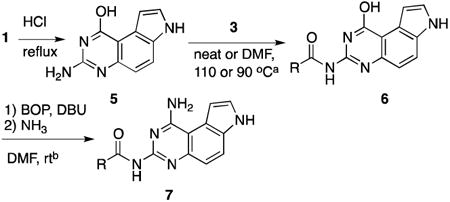
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entry | R | acylating reagent | 6 | yieldc | 7 | yieldc |
| 1 | Me | 3a | 6a | 83 | 7a | 48 |
| 2 | Et | 3b | 6b | 84 | 7b | 44 |
| 3 | Pr | 3c | 6c | 85 | 7c | 37 |
| 4 | iPr | 3d | 6d | 67 | 7d | 40 |
| 5 | TBSO(CH2)4 | 3e | 6e | 62 | 7e | 50 |
| 6 |
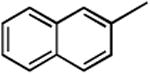
|
3f | 6f | 56 | 7f | 25 |
This step was carried out with compound 5 (1.0 equiv) and an anhydride in neat or an NHS ester (1.5 equiv) in DMF.
This operation was executed with 6 (1.0 equiv), BOP (1.3 equiv) and DBU (1.5 equiv) for 4 h. Then NH3 (7 N in MeOH) was added.
Isolated yields.
