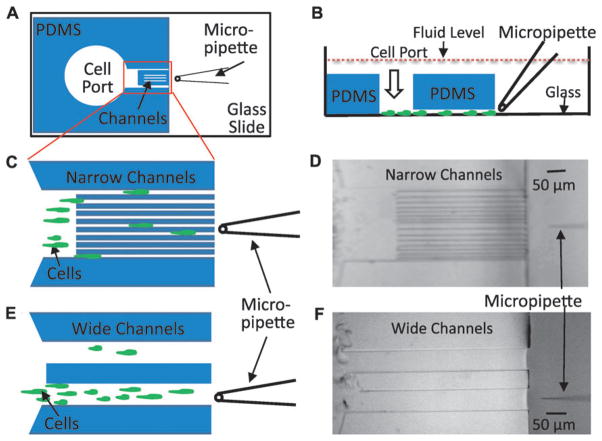
Fig. 1.
The open microfluidic device (OMD) for studying gradient sensing and cell migration. (A) Schematic diagram of an open microfluidic device viewed from the bottom. The PDMS block containing cell port and channels is plasma bonded to the glass coverslip of a one-well chamber. A micropipette filled with chemoattractant is placed in front of the narrow channel region. (B) A side view of the device in 1A depicting cells loaded through the cell port and crawling on the glass surface toward the micropipette. Note that entire device is submerged in buffer solution so that hydrostatic pressure is essentially equivalent on the inside and outside of the device. (C) Magnified cartoon of the narrow channel region with cells crawling between the different-sized channels toward the micropipette placed at the center of the device. In the actual device, there are 16 channels with each size represented 4 times. The channel widths are 6 μm, 6 μm, 8 μm, 8 μm, 10 μm, 10 μm, 12 μm, 12 μm, 6 μm, 6 μm, 8 μm, 8 μm, 10 μm, 10 μm, 12 μm and 12 μm. (D) A bright field image of the device with the narrow channel region described in 1C was obtained using a 10× objective. (E) Schematic of two wide channel regions with a cartoon that depicts cells crawling toward the micropipette. The width of each wide channel is 100 μm. The micropipette is placed at the center of the bottom wide channel. (F) Bright field image acquired using a 10× objective of the device with the wide channels.