Abstract
Background
Rho-associated kinases (ROCKs) have been shown to be involved in the pathogenesis of atherosclerosis. It is clinically important to estimate the degree of ROCK activity in humans. The purpose of this study was to confirm the validity of a leukocyte ROCK parameter as an index of ROCK activity in comparison with vascular response to a ROCK inhibitor.
Methods and Results
We evaluated the ratio of phospho myosin-binding subunit (p-MBS) on myosin light-chain phosphatase to total MBS in peripheral leukocytes by Western blot analysis and forearm blood flow (FBF) response to the ROCK inhibitor fasudil using strain-gauge plethysmography in 36 healthy subjects and 39 patients with cardiovascular diseases. Fasudil (3, 10, 30 μg/min) was infused intra-arterially for 5 minutes at each dose. Leukocyte p-MBS/total-MBS was higher in cardiovascular diseases than in healthy subjects (0.97±0.37 vs. 0.51±0.14; P=0.002). Fasudil increased FBF from 4.9±1.2 to 14.5±5.7 mL/min/100 mL tissue (P<0.0001) in patients with cardiovascular diseases, while fasudil did not alter FBF in healthy subjects. There was a significant relationship between leukocyte p-MBS/total-MBS and maximal FBF response to fasudil in all subjects (r=0.72, P<0.0001). There was also a significant correlation between p-MBS/total-MBS and maximal FBF response to fasudil in patients with cardiovascular diseases (r=0.59, P<0.0001). In healthy subjects, there was no significant correlation between the two parameters.
Conclusions
These findings suggest that assessment of leukocyte ROCK activity is minimally invasive and does not require pharmacologic intervention using ROCK inhibitors. Leukocyte p-MBS/total-MBS may be useful for evaluating ROCK activity in a clinical setting.
Introduction
Small guanosine triphosphate (GTP)-binding protein ras homolog gene family, member A (RhoA) mediates various cellular physiologic functions such as cell proliferation, migration, adhesion, apoptosis and contraction,1-3 all of which may be involved in the pathogenesis of atherosclerosis. Rho-associated kinase (ROCK), the immediate downstream target of RhoA, activates myosin light chain kinase by phosphorylation of the myosin-binding subunit (p-MBS) in myosin light chain phosphatase, which is a downstream target of ROCK, contributing to endothelial dysfunction and cardiovascular disease.4-8 The RhoA/ROCK pathway physiologically plays a key role in atherosclerotic lesion formation, vasoconstriction and myocardial hypertrophy.9-11 Recent clinical evidence has demonstrated that ROCK is significantly activated in patients with coronary vasospasm,12 hypertension,13 and stable effort angina14 and even in current smoking subjects.15,16 ROCK, therefore, is becoming a new therapeutic target in cardiovascular disease.
It is clinically important to estimate the degree of ROCK activity. Recently, several investigators, including us, have evaluated the effects of intra-arterial infusion of the ROCK inhibitor fasudil on forearm blood flow (FBF) or coronary blood flow and the effects of intravenous infusion of fasudil on pulmonary arterial pressure.12-17 The responses to intra-arterial infusion of fasudil should be considered the gold standard for assessing ROCK activity, because the use of an inhibitor of ROCK allowed us to draw more specific conclusions concerning the roles of basal and activated ROCK in atherosclerosis. Inhibition of ROCK in forearm vascular smooth muscle cells (VSMCs) by intra-arterial infusion of fasudil could result in the reduction of phosphorylation of MBS on myosin light chain phosphatase (MLCP) and increase of MCLP activity. Subsequent dephosphorylation of MLC would lead to actomyosin inactivation and consequent relaxation of VSMCs. It has been shown that a method for measuring a specific antibody to p-MBS/total-MBS (t-MBS) in peripheral leukocytes is also useful for assessing ROCK activity.18,19 However, there has been no information on the relationship between leukocyte p-MBS/t-MBS and vascular response to the ROCK inhibitor fasudil.
Therefore, to determine whether measurement of the leukocyte parameter is valid as an index of ROCK activity, we evaluated p-MBS/t-MBS in peripheral leukocytes and FBF response to intra-arterial infusion of fasudil in healthy subjects and patients with cardiovascular diseases.
Methods
Subjects
We studied 75 subjects, including 36 healthy subjects (27 men and 9 women, mean age: 56.1±4.2 years) and 39 patients with cardiovascular disease (29 men and 10 women, mean age: 60.1±3.9 years). The study protocol was approved by the Ethics Committee of Hiroshima University Graduate School of Biomedical Sciences. Informed consent for participation in the study was obtained from all subjects.
Study protocol
A leukocyte parameter of ROCK activity, p-MBS/t-MBS, and forearm vascular responses to fasudil (Asahi Chemical Industry Co Ltd, Tokyo, Japan), a specific ROCK inhibitor, were evaluated in all subjects. The study began at 8:30 AM with the subjects in a fasting condition. A 23-gauge polyethylene catheter (Hokkow Co.) was inserted into the left brachial artery for infusion of each drug and for recording of arterial pressure with an AP-641G pressure transducer (Nihon Kohden Co.) under local anesthesia (1% lidocaine). Another catheter was inserted into the left deep antecubital vein to obtain blood samples. After 30 minutes in the supine position, we measured basal FBF and arterial blood pressure. Then forearm vascular response to fasudil was measured. Fasudil (3, 10, 30 μg/min) was infused intra-arterially for 5 minutes at each dose. FBF was measured during the last 2 minutes of the infusion.
Baseline fasting serum concentrations of total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, creatinine, glucose, and electrolytes were determined and blood for measurement of ROCK activity was obtained after a 30-minute rest period before the study.
Measurement of leukocyte p-MBS/t-MBS
ROCK activity was assayed in peripheral blood leukocytes as the amount of phospho-Thr853 in the MBS of MLCP. Blood was collected at room temperature in heparinized tubes (20 U/mL). After adding an equal volume of 2% dextran, the sample was kept at room temperature for 30 minutes. The supernatant was spun at 1450 rpm for 10 minutes. Red blood cells in the resulting cell pellet were lysed with the addition of water and spun at 1450 rpm for 10 minutes after the addition of Hank's balanced salt solution (Hyclone, Logan, Utah). The resulting leukocyte pellet was resuspended in medium 199 (Sigma Chemical Co) and counted using a hematocytometer. Cells were fixed in 10% trichloroacetic acid and 10 mmol/L dichlorodiphenyltrichloroethane. After centrifugation, the cell pellets were stored at -80°C for Western blot analysis. Cells pellets were dissolved in 10 μL of 1 mol/L Tris base and then mixed with 100 μL of extraction buffer (8 mol/L urea, 2% sodium dodecyl sulfate, 5% sucrose, and 5% 2-mercaptoethanol). Equal amounts of cell extracts were subjected to 7.5% SDS-PAGE and transferred to nitrocellulose membranes. NIH 3T3 cell lysates were used as a positive control and to standardize the results of Western blot analyses from several membranes. After serum starvation for 20 hours, confluent cells were stimulated with 10 μmol/L lysophosphatidic acid for 10 minutes and then subsequently fixed and harvested in 10% trichloroacetic acid and 10 mmol/L dichlorodiphenyltrichloroethane. Following centrifugation at 1450 rpm for 10 minutes at 4°C, precipitates were dissolved in 10 μL of 1 mol/L Tris base and mixed with 100 μL of extraction buffer. An equal volume of positive control cell lysate was used for each gel. Membranes were incubated with rabbit anti–phospho-specific Thr853–MBS polyclonal antibody (Biosource Invitrogen, Carlsbad, California) or rabbit anti-MBS polyclonal antibody (Covance Laboratories, Evansville, Ind), or antiactin monoclonal antibody (Sigma). Bands were visualized using the ECL system (Amersham-Pharmacia Co., UK). Images were captured using Adobe Photoshop, and the band intensities were quantified using National Institutes of Health Image 1.61. ROCK activity was expressed as the ratio of phospho-Thr853–MBS in each sample to phosphoThr853–MBS in each positive control divided by MBS in each sample per MBS in each positive control.
Measurements of FBF
FBF was measured with a mercury-filled Silastic strain-gauge plethysmography (EC-5R, D.E. Hokanson, Inc.), as previously described.20,21 Briefly, a straingauge was attached to the upper part of the left arm and connected to a plethysmography device, and was supported above the right atrium. A wrist cuff was inflated to a pressure of 50 mmHg above the systolic blood pressure 1 min before each measurements and throughout the measurement of FBF to exclude the hand circulation from the measurements. The upper arm-congesting cuff was inflated to 40 mmHg for 7 seconds during each 15-second cycle using a rapid cuff inflator (EC-20; DE Hokanson, Inc.) to occlude venous outflow from the arm. The FBF output signal was transmitted to a recorder (U-228; Advance Co.). FBF is expressed as milliliters per minute per 100 milliliters of forearm tissue volume. The mean of 4 plethysmographic measurements was used for the analysis of FBF at baseline and during the administration of fasudil.
Statistical analysis
Results are presented as mean ± SD. Unpaired Student's t test was used for comparison of mean values of parametric continuous variables between the cardiovascular disease and healthy groups. Because of asymmetric distribution, value for ROCK activity was analyzed by nonparametric methods. For nonparametric analysis, we used Mann-Whitney U test to evaluate the difference of levels between groups. Comparisons between the two groups with respect to changes in parameters were performed with adjusted means on an ANCOVA, with baseline data used as the covariates. Comparisons of dose-response curves of parameters during the infusion of fasudil were analyzed by ANOVA for repeated measures with Bonferroni correction. All reported p values were 2-sided, and a p value of <0.05 was considered statistically significant. The data were analyzed using the software package StatView V (SAS Institute) and Super ANOVA (Abacus Concepts, Berkley, California).
Results
Clinical characteristics
Baseline clinical characteristics in the 36 healthy subjects and 39 patients with cardiovascular diseases are summarized in Table 1. Systolic and diastolic blood pressures and serum concentrations of total cholesterol, triglycerides, LDL cholesterol and glucose were higher and HDL cholesterol was lower in patients with cardiovascular diseases than in healthy subjects. The other parameters were similar in the two groups.
Table 1. Clinical Characteristics of the Healthy Subjects and Patients with Cardiovascular Diseases.
| Variable | Healthy subjects (n=36) | Cardiovascular diseases (n=39) |
|---|---|---|
| Body mass index (kg/m2) | 24.3 ± 2.7 | 24.6 ± 2.9 |
| Systolic blood pressure (mm Hg) | 114.8 ± 10.2 | 149.7 ± 13.2* |
| Diastolic blood pressure (mm Hg) | 68.8 ± 8.5 | 96.5 ± 10.1* |
| Heart rate (bpm) | 68.9 ± 7.7 | 70.6 ± 8.9 |
| Total cholesterol (mmol/L) | 4.77 ± 0.69 | 5.32 ± 0.91* |
| Triglycerides (mmol/L) | 1.11 ± 0.38 | 1.57 ± 0.47* |
| HDL cholesterol (mmol/L) | 1.45 ± 0.31 | 1.22 ± 0.39* |
| LDL cholesterol (mmol/L) | 3.17 ± 0.57 | 4.12 ± 0.79* |
| Glucose (mmol/L) | 4.6 ± 0.3 | 5.2 ± 0.7* |
| Creatinine (μmol/L) | 82.5 ± 19.6 | 93.2 ± 27.5 |
| FBF (mL/min/100 mL tisuure) | 5.0 ± 1.3 | 4.9 ± 1.2 |
| Smoker (n) | 0 | 16 |
HDL indicates high density lipoprotein; LDL, low density lipoproteine, FBF, forearm blood flow.
P<0.05 versus healthy subjects.
Leukocyte p-MBS/t-MBS
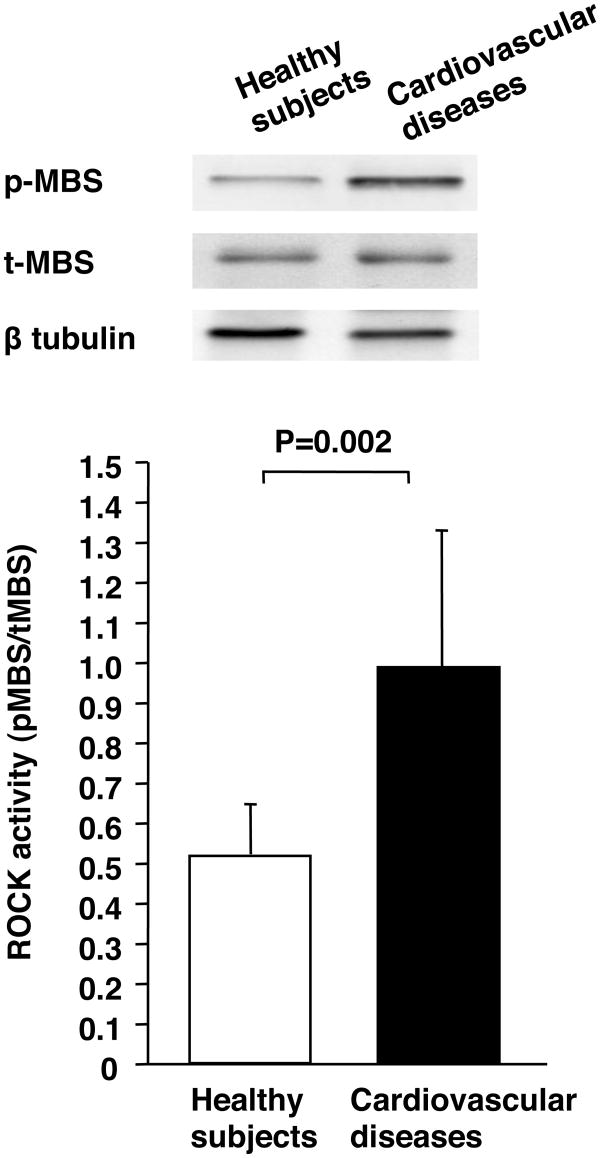
Leukocyte p-MBS/t-MBS was significantly higher in patients with cardiovascular diseases than in healthy subjects (0.97±0.37 vs. 0.51±0.14, P=0.002) (Figure 1). Protein expression of t-MBS was similar in the two groups (0.91±0.34 vs. 1.04±0.41).
Figure 1.
Rho-associated kinase (ROCK) activity in healthy subjects and patients with cardiovascular diseases. Western blot analysis for phospho-myosin-binding subunit (p-MBS), total-myosin-binding subunit (t-MBS), and β tublin (upper). ROCK activity (p-MBS/t-MBS) in healthy subjects and patients with cardiovascular diseases (bottom).
FBF response to fasudil
The intra-arterial infusion of fasudil significantly increased FBF in patients with cardiovascular diseases, while fasudil did not alter FBF in healthy subjects. The FBF response to fasudil was significantly higher in patients with cardiovascular diseases than in healthy subjects (maximal FBF: 14.5±5.7 versus 6.3±1.5 mL/min per 100 mLtissue; P=0.02, Figure 2). No significant change was observed in arterial blood pressure or heart rate with intra-arterial infusion of fasudil.
Figure 2.
Forearm blood flow response to fasudil in healthy subjects and patients with cardiovascular diseases. *P<0.05 vs. baseline in the same group. †P<0.05 vs. healthy subjects at the same dose.
Relationship between FBF response to fasudil and leukocyte ROCK activity
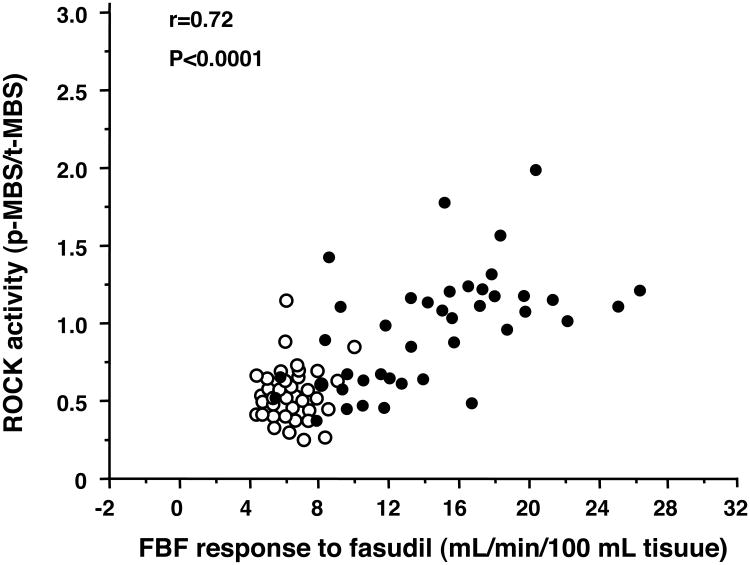
There was a significant relationship between leukocyte p-MBS/total-MBS and maximal FBF response to fasudil in all subjects (r=0.72, P<0.0001) (Figure 3). There was also a significant correlation between p-MBS/total-MBS and maximal FBF response to fasudil in patients with cardiovascular diseases (r=0.59, P<0.0001). In healthy subjects, there was no significant correlation between the two parameters.
Figure 3.
Relationship between leukocyte phospho-myosin-binding subunit (p-MBS)/total-myosin-binding subunit (t-MBS) and maximal forearm blood flow (FBF) response to fasudil in healthy subjects (open circle) and patients with cardiovascular diseases (closed circle).
Discussion
The present study demonstrated that leukocyte p-MBS/t-MBS and FBF response to fasudil were higher in patients with cardiovascular diseases than in healthy subjects. There was a significant correlation between leukocyte p-MBS/t-MBS, a noninvasive measurement, and the FBF response to intra-arterial infusion of fasudil in all subjects. Leukocyte p-MBS/t-MBS significantly correlated with FBF response to fasudil in patients with cardiovascular diseases but not in healthy subjects.
ROCK inhibitors such as fasudil and Y-27632 have been used in previous studies to assess ROCK activity in vitro and in vivo12-16,22 In human studies also, the response to intra-arterial or intravenous infusion of ROCK inhibitors should be considered the gold standard in assessing ROCK activity, since the use of antagonists to inhibit ROCK activity allowed us to draw more specific conclusions concerning the roles of basal and stimulated ROCK activity. Indeed, it has been confirmed that ROCK activity assessed by vascular response to the ROCK inhibitor fasudil is enhanced in vasospastic angina,12 hypertension,13 angina pectoris,14 pulmonary hypertension,17 heart failure,23 and stroke.24 However, the invasive method using an intra-arterial infusion of drugs is time-consuming and is a burden for subjects. In the present study, leukocyte p-MBS/t-MBS significantly correlated with maximal FBF response to the ROCK inhibitor fasudil (30 μg/min for 5 minutes). In addition, it has been shown that statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, inhibit ROCK activity assessed by leukocyte p-MBS/t-MBS through a cholesterol-independent effect in patients with dyslipidemia and atherosclerosis.25-27 These findings suggest that evaluating leukocyte p-MBS/t-MBS is a useful alternative to intra-arterial infusion of ROCK inhibitors in assessment of ROCK activity and reflects ROCK activity in humans. A noninvasive method for measuring leukocyte p-MBS/t-MBS is simple and reproducible and does not have adverse effects.
In the present study, although we found a significant relationship between leukocyte p-MBS/t-MBS and the FBF response to fasudil in patients with cardiovascular diseases, leukocyte p-MBS/t-MBS did not correlate with the FBF response to fasudil in healthy subjects. An obvious difference between leukocyte p-MBS/t-MBS FBF responses to fasudil in healthy subjects and patients with cardiovascular diseases has been shown. In healthy subjects, intra-arterial infusion of fasudil did not alter FBF. Our results are consistent with results of previous studies showing that vascular responses to fasudil were not augmented in healthy subjects.13 These findings suggest that ROCK might not be activated in subjects without cardiovascular risk factors. It is likely that both methods of measuring leukocyte p-MBS/t-MBS and vascular response to fasudil reflect ROCK activity in a pathophysiological state.
It is well known that peripheral blood leukocytes per se play an important role inthe cause, maintenance, and development of atherosclerosis. Bao et al.28 showed that treatment with the ROCK inhibitor Y-27632 reduced accumulation of neutrophils by 45% in the ischemic myocardium. In addition, Wolfrum et al.29 showed that fasudil decreased leukocyte recruitment and adhesion to the endothelium after ischemic reperfusion injury in wild-type butnot endothelial nitric oxide knockout mice. Although there are no experimental data on the role of ROCK activity in the leukocyte function in offspring or established atherosclerosis, the enhancement of ROCK activity assessed by leukocyte p-MBS/t-MBS in cardiovascular diseases may cause leukocyte activation and enhanced leukocyte infiltration into the vascular wall, leadingto progression of atherosclerosis. Interestingly, leukocyte ROCK activity has been shown to correlate with endothelial dysfunction in patients with coronary artery disease.30
Limitation
We selected measurement of leukocyte p-MBS/t-MBS as a noninvasive method for assessing ROCK activity, since peripheral leukocytes are able to be simply and feasibly obtained. Measurement of p-MBS/t-MBS in endothelial cells and vascular smooth muscle cells from humans would enable more specific confirmation of vascular ROCK activity. Unfortunately, we can not easily obtain these samples. In the present study, leukocyte p-MBS/t-MBS significantly correlated with FBF response to fasudil. Therefore, we consider that measurement of leukocyte p-MBS/t-MBS is also an index of vascular ROCK activity.
This study is not a randomized controlled trial. Although a randomized and/or interventional clinical trial would reveal further importance of leukocyte ROCK activity, this study showed a substantial relationship of leukocyte ROCK activity with vascular ROCK activity.
Smoking is a predictor of leukocyte ROCK activity. Therefore, in the present study, we excluded smokers in healthy subjects. We have reanalyzed data after excluding smokers. Leukocyte p-MBS/t-MBS was significantly higher in patients with cardiovascular diseases than in healthy subjects (0.86±0.39 vs. 0.51±0.14, P=0.01). The FBF response to fasudil was significantly higher in patients with cardiovascular diseases than in healthy subjects (maximal FBF: 12.9±6.1 versus 6.3±1.5 mL/min per 100 mL tissue; P=0.03). There was a significant relationship between leukocyte p-MBS/total-MBS and maximal FBF response to fasudil in all subjects (r=0.69, P<0.0001). There was also a significant correlation between p-MBS/total-MBS and maximal FBF response to fasudil in patients with cardiovascular diseases (r=0.51, P<0.0001). After excluding smokers, we confirmed the validity of a leukocyte ROCK parameter as an index of ROCK activity in patients with cardiovascular diseases. Previous studies have also reported that ROCK is activated in patients with cardiovascular diseases as well as cardiovascular risk factors.12,13,15,16,22,23,30 However, we cannot deny the possibility that an increase in ROCK activity may be due to smoking and other cardiovascular risk factors and not cardiovascular diseases per se.
In conclusion, from a clinical perspective, it is important to estimate the grade of basal condition of ROCK and of improvement in enhanced ROCK activity. A noninvasive method for measuring leukocyte p-MBS/t-MBS would also be useful for assessing ROCK activity. Measurement of p-MBS/t-MBS in peripheral leukocytes is minimally invasive and does not require pharmacologic intervention (e.g., intra-arterial or intravenous infusion of ROCK inhibitors) and may be useful as a novel predictor of cardiovascular disease in a clinical setting.
Acknowledgments
We thank Megumi Wakisaka and Satoko Michiyama for their excellent secretarial assistance.
Sources of Founding: This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (1559075100 and 1859081500), and the National Institutes of Health (HL052233 and HL08187).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. James K Liao is a consultant for Asahi-Kasei Pharmaceutical, Inc.
References
- 1.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 2.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 3.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 4.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 5.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 6.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz AR, Parsons JT. Cell migration--movin' on. Science. 1999;286:1102–1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- 9.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res. 2003;93:884–888. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 10.Seasholtz TM, Wessel J, Rao F, Rana BK, Khandrika S, Kennedy BP, Lillie EO, Ziegler MG, Smith DW, Schork NJ, et al. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: role of heredity. Hypertension. 2006;47:937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 11.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/- haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 13.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 14.Shimokawa H, Hiramori K, Iinuma H, Hosoda S, Kishida H, Osada H, Katagiri T, Yamauchi K, Yui Y, Minamino T, Nakashima M, Kato K. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol. 2002;40:751–761. doi: 10.1097/00005344-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Noma K, Higashi Y, Jitsuiki D, Hara K, Kimura M, Nakagawa K, Goto C, Matsuura H, Oshima T, Yoshizumi M, Chayama K. Smoking activates rho-kinase in smooth muscle cells of forearm vasculature in humans. Hypertension. 2003;41:1102–1105. doi: 10.1161/01.HYP.0000067062.92836.9E. [DOI] [PubMed] [Google Scholar]
- 16.Noma K, Goto C, Jitsuiki D, Ueda K, Kimura M, Nishioka K, Umemura T, Nakagawa K, Oshima T, Yoshizumi M, Chayama K, Higashi Y. Smoking, endothelial function, and Rho-kinase in humans. Arterioscler Thromb Vasc Biol. 2005;25:2630–2635. doi: 10.1161/01.ATV.0000189304.32725.bd. [DOI] [PubMed] [Google Scholar]
- 17.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 18.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 19.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 20.Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Ssaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 21.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial Function and Oxidative Stress in Renovascular Hypertension. N Engl J Med. 2002;346:1954–1962. doi: 10.1056/NEJMoa013591. [DOI] [PubMed] [Google Scholar]
- 22.Noma K, Goto C, Nishioka K, Jitsuiki D, Umemura T, Ueda K, Kimura M, Nakagawa K, Oshima T, Chayama K, Yoshizumi M, Liao JK, Higashi Y. Roles of rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49:698–705. doi: 10.1016/j.jacc.2006.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishi T, Hirooka Y, Masumoto A, Ito K, Kimura Y, Inokuchi K, Tagawa T, Shimokawa H, Takeshita A, Sunagawa K. Rho-kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation. 2005;111:2741–2747. doi: 10.1161/CIRCULATIONAHA.104.510248. [DOI] [PubMed] [Google Scholar]
- 24.Feske SK, Sorond FA, Henderson GV, Seto M, Hitomi A, Kawasaki K, Sasaki Y, Asano T, Liao JK. Increased leukocyte ROCK activity in patients after acute ischemic stroke. Brain Res. 2009;1257:89–93. doi: 10.1016/j.brainres.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawlings R, Nohria A, Liu PY, Donnelly J, Creager MA, Ganz P, Selwyn A, Liao JK. Comparison of effects of rosuvastatin (10 mg) versus atorvastatin (40 mg) on rho kinase activity in caucasian men with a previous atherosclerotic event. Am J Cardiol. 2009;103:437–441. doi: 10.1016/j.amjcard.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nohria A, Prsic A, Liu PY, Okamoto R, Creager MA, Selwyn A, Liao JK, Ganz P. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis. 2009;205:517–521. doi: 10.1016/j.atherosclerosis.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119:131–138. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, Ma XL, Willette RN, Yue TL. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res. 2004;61:548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99:1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]