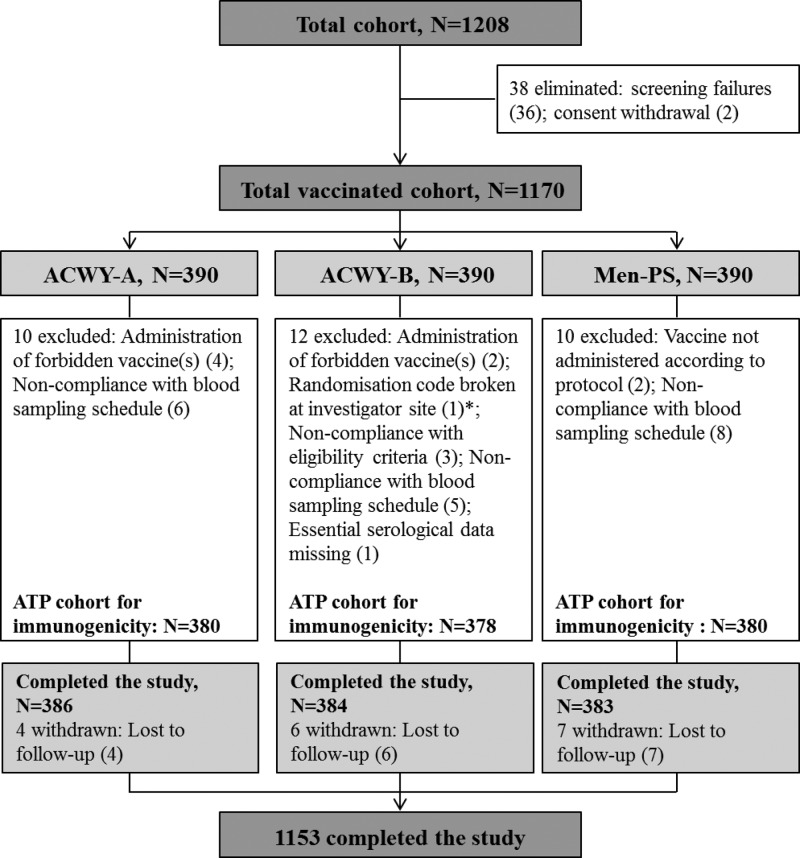
Fig 1.
Subject flow chart showing the number of subjects enrolled, the number who completed the study, and the reasons for exclusion from the ATP cohort for immunogenicity. *, the randomization code was broken at the investigator site (to determine which vaccine had been administered) for one subject because the subject experienced a serious adverse event (blighted ovum) that was assessed by the investigators as possibly being related to the study vaccine. ACWY-A, subjects vaccinated with MenACWY-TT lot A; ACWY-B, subjects vaccinated with MenACWY-TT lot B; Men-PS, subjects vaccinated with Men-PS; ATP, according-to-protocol; N, total number of subjects. Numbers in parentheses indicate numbers of subjects.