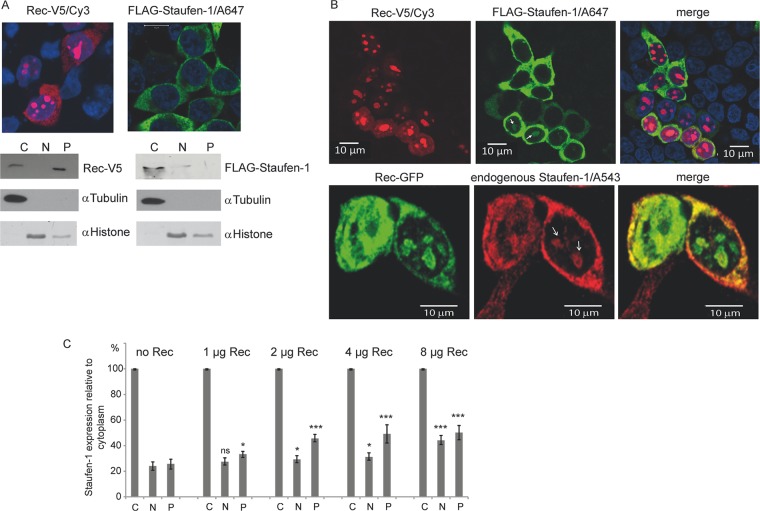
Fig 3.
Subcellular localization of Rec and Staufen-1. HEK 293T cells were transfected to express Rec-V5, FLAG–Staufen-1, or both proteins. (A) Immunofluorescence (top) and cell fractionation studies (bottom) of cells expressing each protein alone. The proteins were separated into cytoplasmic (C), nuclear (N), and insoluble pellet (P) fractions. Fractionation efficiency was confirmed with antibodies against tubulin as a cytoplasmic marker and histone as a nuclear protein. (B) Immunofluorescence of cells transfected with Rec-V5 and FLAG–Staufen-1 or Rec-GFP only. Colocalization of Rec with ectopically expressed (top) or endogenous (bottom) Staufen-1 in the cytosol and in bulky nucleoli of some cells is evident (indicated by white arrows). A647, Alexa 647. (C) Analysis of the Rec-dependent localization of endogenous Staufen-1 by cell fractionation into the cytoplasmic fraction, soluble nuclear fraction, and insoluble pellet from dot blot experiments using the Odyssey imaging system for quantification. The signal of the cytoplasmic fraction of each sample was set at 100% to estimate the change in endogenous Staufen-1 distribution in the other fractions. P values were calculated by comparing the signals from either the nuclear fractions or the protein pellets with those from cells without Rec. P values are indicated as ∗ (P < 0.05), ∗∗ (P < 0.01), ∗∗∗ (P < 0.001), and ns (not significant).