Abstract
The influenza A virus genome comprises eight single-stranded negative-sense RNA segments (vRNAs). All eight vRNAs are selectively packaged into each progeny virion via so-called segment-specific genome-packaging signal sequences that are located in the noncoding and terminal coding regions of both the 3′ and the 5′ ends of the vRNAs. However, it remains unclear how these signals ensure that eight different vRNAs are packaged. Here, by using a reverse genetics system, we demonstrated that, in the absence of the other seven vRNAs, a recombinant NP vRNA bearing only a reporter gene flanked by the noncoding NP regions was incorporated into virus-like particles (VLPs) as efficiently as a recombinant NP vRNA bearing the reporter gene flanked by the complete NP packaging signals (i.e., the noncoding sequences and the terminal coding regions). Viruses that comprised a recombinant NP vRNA whose packaging signal was disrupted, and the remaining seven authentic vRNAs, did not undergo multiple cycles of replication; however, a recombinant NP vRNA with only the noncoding regions was readily incorporated into VLPs, suggesting that the packaging signal as currently defined is not necessarily essential for the packaging of the vRNA in which it resides; rather, it is required for the packaging of the full set of vRNAs. We propose that the 3′ and 5′ noncoding regions of each vRNA bear a virion incorporation signal for that vRNA and that the terminal coding regions serve as a bundling signal that ensures the incorporation of the complete set of eight vRNAs into the virion.
INTRODUCTION
The genome of influenza A virus is segmented into eight single-stranded negative-sense RNAs. While this genome segmentation provides influenza viruses with a considerable evolutionary advantage through gene reassortment, it complicates the genome packaging of the progeny virions. For decades, the genome-packaging mechanism had been controversial (1, 2); however, recent studies clearly favor the selective packaging model in which influenza virions selectively incorporate a complete set of eight distinct vRNAs. The selective packaging model is experimentally supported by (i) electron microscopic studies showing that eight ribonucleoprotein complexes (RNPs), which are composed of each vRNA, multiple copies of nucleoprotein (NP), and an RNA-dependent RNA polymerase complex, are packaged into progeny virions in a characteristic arrangement (3–6), (ii) a single-molecule fluorescent in situ hybridization analysis and an analysis of using artificial vRNAs encoding different fluorescent reporter proteins showing that most virions contain only one copy of each vRNA (7, 8), and (iii) reverse genetics studies demonstrating that segment-specific packaging signal sequences are necessary for efficient packaging of vRNAs into progeny virions (9–14). It is now widely accepted that the majority of each influenza A virion incorporates one copy of each of the eight different vRNAs (15).
Genome packaging signals have been found in all eight influenza A virus vRNAs (1, 9–16). Each packaging signal is bipartite, being located in the noncoding and terminal coding regions of both the 3′ and 5′ ends of each vRNA. Each packaging signal is unique to each vRNA, and it is this uniqueness that probably leads to the discriminate incorporation of the individual vRNAs into progeny virions. The introduction of mutations into the genome-packaging signal markedly reduces the packaging efficiency of the mutated vRNA into progeny virions (16–24), as well as that of the other vRNAs (14, 18, 21), demonstrating the significance of the genome-packaging signal for vRNA incorporation into virions. Recent electron microscopic and biochemical studies suggest that there should be interactions among the vRNAs, most likely in the form of RNPs (5, 6, 25); however, it remains unclear how these packaging signals function during vRNA incorporation into virions. Here, we used reverse genetics to clarify the role of the packaging signals. We found that the so-called genome-packaging signal serves two distinct functions: the virion incorporation of RNP and the bundling of multiple RNPs.
MATERIALS AND METHODS
Cells and virus.
Human embryonic kidney (HEK) 293T cells were maintained in Dulbecco modified Eagle medium containing 10% fetal calf serum (FCS). Madin-Darby canine kidney (MDCK) cells were maintained in Eagle minimal essential medium (MEM) containing 5% newborn calf serum. All cells were maintained at 37°C under 5% CO2. Influenza A/WSN/33 (H1N1; referred to as WSN) was propagated in MDCK cells.
Plasmid construction.
pPolI NP(0)GFP(0) and pPoII NP(120)GFP(120) were described previously (15). To generate pPolI N0-FLAG NP, the open reading frame (ORF) of pPolI-WSN-NP was replaced with the NFLAG NP gene from pCAGGS NFLAG NP. Briefly, the NP ORF in pPolI-WSN-NP was eliminated by back-to-back PCR with primers containing BsmBI sites. Using the BsmBI sites, the NFALG NP gene containing BsmBI sites at both ends, which was generated by PCR, was cloned. To generate pPolI N49ATC-FLAG NP, three ATG codons in the 49 nucleotides at the 5′ end of the NP cDNA were changed by using site-directed mutagenesis. The remaining region from the 50th nucleotide to the end of the NP ORF was replaced with the NFLAG NP gene as described above. pPolI N49ATCinv-FLAG NP was generated by back-to-back PCR using pPolI N49ATC-FLAG NP as a template, which was digested with BsmBI and self-ligated. The primers used were as follows: N49ATCinvF (5′-CACACACGTCTCTCTAGCAAACCACGGAAACCAGCGGCGATGGACTACAAGGACGACGACGACAAG-3′) and N49ATCinvR (5′-CACACACGTCTCGCTAGAATGCTTGTCTAGCTCTGACTAGGATTTCGATGTCACTCTGTGAGTGATTATCCACACACGTCTCGCTAGAATGCTTGTCTAGCTCTGACTAGCACACACGTCTCTCTAGCAAACCACGGAAACCAGCGGCG-3′). All constructs were sequenced to confirm the absence of undesired substitutions.
Reverse genetics.
Viruses and virus-like particles (VLPs) were generated as described previously (15). Briefly, 293T cells (106 cells per 3.5-cm culture dish) were transfected with a cocktail of 18 plasmids (0.1 μg of each of 8 pPolI plasmids for vRNA generation, 1 μg of PB1, PB2, PA, NP, HA, NA, NS1, and NS2 expression plasmids, 2 μg of M1 expression plasmid, and 0.1 μg of M2 expression plasmid) by using TransIT293 (PanVera). To generate VLPs with a single vRNA, we used 0.8 μg of a single pPolI plasmid instead of the 8 pPolI plasmids. At 24 h posttransfection, the supernatants containing viruses or VLPs were harvested after a 30-min incubation at 37°C with TPCK (tolylsulfonyl phenylalanyl chloromethyl ketone)-trypsin (final concentration, 0.5 μg/ml). Cell debris was removed by centrifugation.
Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blots were performed according to standard procedures. Plasmid-transfected 293T cells were lysed in SDS sample buffer, separated on a 4 to 20% gradient Tris-glycine gel, and then transferred to a polyvinylidene difluoride membrane. Blots were probed with a mouse anti-FLAG M2 monoclonal antibody (MAb; Sigma), rabbit anti-WSN R309 serum, or a mouse anti-β actin MAb (Sigma), followed by horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG or HRP-conjugated donkey anti-rabbit IgG (GE Healthcare). They were then visualized by using ECL Plus Western blotting detection reagents (GE Healthcare) according to the manufacturer's instructions.
Flow cytometry.
MDCK cells were infected with VLPs containing NP(0)GFP(0) vRNA or NP(120)GFP(120) vRNA, together with a helper A/WSN/33 virus to supply intact NP protein in trans. At 8 h postinfection, the cells were rinsed with phosphate-buffered saline (PBS) containing 0.02% EDTA and trypsinized to prepare a single cell suspension, which contained 2% FCS and 0.01% sodium azide. Green fluorescent protein (GFP) expression was analyzed in an FL1 detector of FACSCalibur (Becton Dickinson).
Plaque assay.
MDCK cells infected with viruses were cultured in MEM containing 0.3% bovine serum albumin, 0.5 μg of TPCK-trypsin/ml, and 1% agarose for 48 h. Plaques were counted after crystal violet staining.
VLP infection assay.
MDCK cells were infected with viruses or VLPs, together with a helper WSN virus. At 7 h postinfection, the cells were fixed with 4% paraformaldehyde in phosphate buffer for 15 min at room temperature, permeabilized with PBS containing 0.2% Triton X-100 for 10 min, and incubated with an anti-FLAG M2 MAb or rabbit anti-WSN R309 serum. The cells were detected by using a Vectastain Elite ABC kit (Vector Laboratories) and 3,3′-diaminobenzidine (DAB) staining.
RESULTS
The noncoding regions of NP vRNA are sufficient for its incorporation into VLPs.
The packaging signal for NP vRNA is reported to comprise 60 nucleotides at the 3′ end and 120 nucleotides at the 5′ end of the coding region, together with the noncoding regions, of the NP vRNA (15). To confirm this report, we used two plasmids to express mutant NP vRNAs: NP(0)GFP(0) vRNA and NP(120)GFP(120) vRNA (Fig. 1A). NP(0)GFP(0) vRNA contains a GFP-coding sequence flanked by the 3′ and 5′ noncoding regions of NP vRNA, whereas the NP(120)GFP (120) vRNA contains a GFP-coding sequence flanked by the terminal 120 nucleotides of the coding region and the noncoding regions of NP vRNA. We transfected 293T cells with a plasmid for the transcription of the mutant NP-GFP vRNA, seven plasmids for the production of the remaining vRNAs, and ten plasmids for the expression of the viral proteins (i.e., PB2, PB1, PA, HA, NP, NA, M1, M2, NS1, and NS2) and harvested the supernatants containing VLPs at 24 h posttransfection. MDCK cells were then infected with the VLPs produced with NP(0)GFP(0) vRNA or NP(120)GFP(120) vRNA, together with a helper WSN virus (at a multiplicity of infection of 1) to provide functional NP protein in trans. At 8 h postinfection, the GFP-expressing cells were counted. Of 20,000 cells counted, 28% expressed GFP when infected with VLPs produced with NP(120)GFP(120) vRNA. On the other hand, infection of cells with the VLPs produced with NP(0)GFP(0) vRNA resulted in GFP expression in only 2% of the cells (Fig. 1B). Thus, consistent with the previous report (15), the incorporation efficiency of the NP(120)GFP(120) vRNA was significantly higher than that of the NP(0)GFP(0) vRNA (Fig. 1B, left), confirming that the coding regions within the packaging signal sequences of NP vRNA are required for the efficient packaging of the NP vRNA into VLPs under these conditions.
Fig 1.
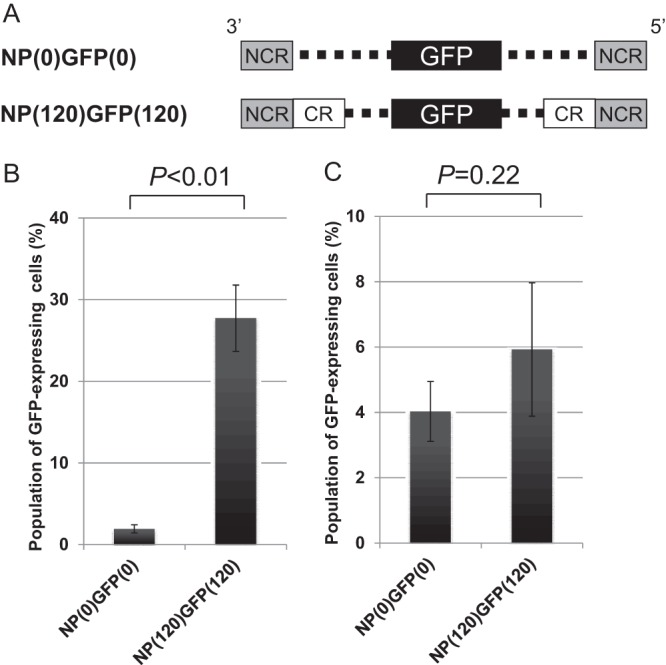
Effect of the packaging signal on the incorporation efficiency of recombinant NP vRNA in the presence or absence of the other seven vRNAs. (A) Schematic diagram of the recombinant NP vRNA. The noncoding (NCR) and coding (CR) regions are represented by gray and white boxes, respectively; the dashed lines indicate nucleotides deleted from the NP coding region. The GFP ORF was inserted in frame with the NP ORF, as indicated by the black boxes. (B and C) The incorporation efficiencies of the recombinant NP vRNAs were determined as described in Materials and Methods in the presence (eight segments) (B) or absence (one segment) (C) of the remaining seven vRNAs. Statistical analysis was performed using a paired Student t test.
Next, to examine whether the packaging signal is also involved in the efficient incorporation of the NP vRNA into VLPs in the absence of the remaining seven vRNAs, we transfected 293T cells with a plasmid for the production of NP(0)GFP(0) vRNA or NP(120)GFP(120) vRNA and ten plasmids for the expression of the viral proteins under the same conditions. MDCK cells were then infected with the VLPs, together with a helper WSN virus, and the GFP-expressing cells were counted. Interestingly, the incorporation efficiency of NP(0)GFP(0) vRNA was not significantly different from that of NP(120)GFP(120) vRNA (Fig. 1C), suggesting that, in the absence of the remaining seven vRNAs, the coding region within the packaging signal of NP vRNA does not contribute to efficient NP vRNA incorporation into VLPs and that the noncoding regions of the NP vRNA are sufficient for the incorporation of the NP vRNA.
The packaging signal of NP vRNA is necessary for the production of replication-competent viruses containing eight different vRNAs.
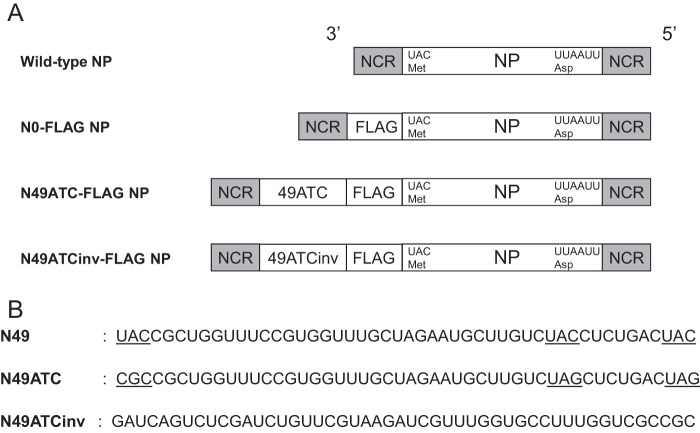
The packaging signal consists of a stretch of noncoding region and the adjacent coding region at the 3′ and 5′ end of each vRNA. To investigate the importance of the packaging signal sequence in virus production, we made three plasmids to generate recombinant NP vRNAs: N0-FLAG NP, N49ATC-FLAG NP, and N49ATCinv-FLAG NP vRNAs (Fig. 2A), all of which express the NP protein fused to a FLAG epitope at its N terminus (NFLAG-NP). The N0-FLAG NP vRNA contains 27 nucleotides that encode a methionine residue and a FLAG peptide between the noncoding region (NCR) and the NP-coding region (Fig. 2A). The N49ATC-FLAG vRNA contains the same 27 nucleotides plus an additional 49 nucleotides (49ATC) that correspond to the 49 nucleotides at the 5′ end of the NP ORF, between the NCR and the NP-coding region (Fig. 2A). To prevent the expression of NP protein from the inserted 49 nucleotides, 3 UAC codons (ATG codons in the mRNA sense) were changed into CGC or UAG (Fig. 2B, underlines). The N49ATCinv-FLAG vRNA contains the 27 nucleotides plus the additional 49 nucleotides with the sequence inverted (49ATCinv) (Fig. 2B). Of these recombinant NP vRNAs, only the N49ATC-FLAG vRNA possesses most of the packaging signal sequence at the 3′ end. The packaging signal sequence of the N0-FLAG NP vRNA and N49-FLAGinv NP vRNA at the 3′ end is separated by the inserted nucleotides that encode the FLAG peptide and by those that encode 49ATCinv and the FLAG peptide, respectively, such that both recombinant NP vRNAs lack packaging signals.
Fig 2.
Schematic diagram of the recombinant NP vRNAs. (A) N0-FLAG NP vRNA contains 27 nucleotides that encode a methionine residue and a FLAG epitope (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys) between the NCR and NP ORF. N49ATC-FLAG NP vRNA contains 76 nucleotides for N49ATC, methionine, and the FLAG epitope. N49ATCinv-FLAG NP vRNA contains 76 nucleotides for N49ATCinv, methionine, and the FLAG epitope. (B) N49 corresponds to the 49 nucleotides of the terminal coding sequence at the 3′ end of the wild-type NP vRNA, in which three UAC (AUG in the mRNA sense) codons are underlined. In N49ATC, the first UAC codon was changed to a CGC codon, and the second and third UAC codons were replaced with UAG codons. N49ATCinv is the N49ATC sequence in the inverse orientation.
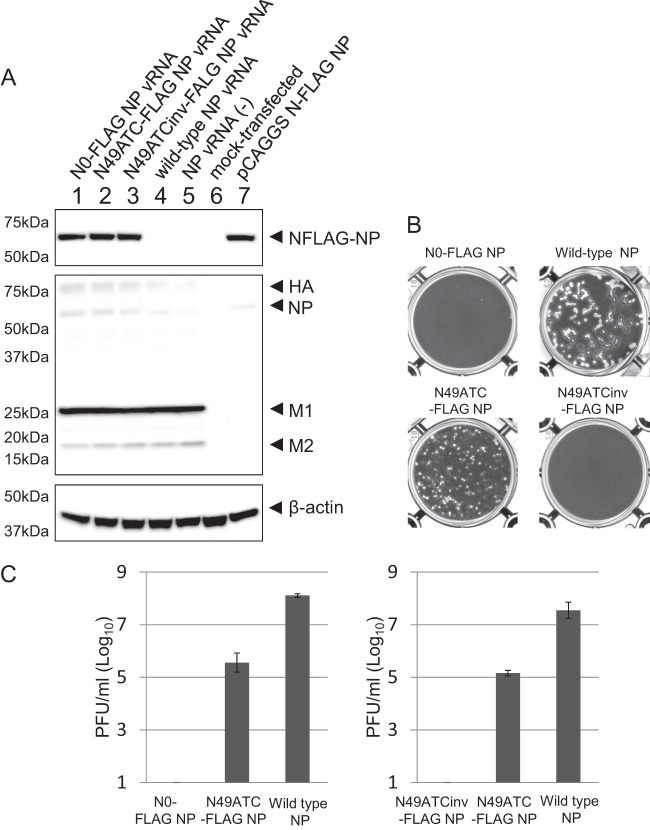
To examine whether N0-FLAG NP vRNA, N49ATC-FLAG NP vRNA, or N49ATCinv-FLAG NP vRNA is incorporated into virions, together with the remaining seven vRNAs, to generate replication-competent virions, we transfected 293T cells with a plasmid for the expression of wild-type NP, N0-FLAG NP, N49ATC-FLAG NP, or N49ATCinv-FLAG NP vRNA, together with the seven plasmids for the expression of the remaining seven vRNAs and with ten plasmids for the expression of viral proteins. Western blot analysis with an anti-FLAG monoclonal antibody and an anti-WSN polyclonal antibody demonstrated that NFLAG-NP and the other viral proteins were expressed at similar levels among the plasmid-transfected cells (Fig. 3A). At 24 h posttransfection, the supernatants containing the viruses were harvested and subjected to plaque assays. Although the virus production with N49ATC-FLAG NP vRNA in 293T cells was less efficient than that with wild-type NP vRNA, both viruses formed plaques (Fig. 3B and C), indicating that N49ATC-FLAG NP vRNA was incorporated into virions, together with the remaining seven vRNAs and maintained during repeated cycles of replication, resulting in replication-competent virions. In contrast, viruses generated with N0-FLAG NP vRNA or N49ATCinv-FLAG NP vRNA failed to form plaques (Fig. 3B and C) and therefore were replication deficient. We confirmed the functionality of the NFLAG-NP expressed from these three recombinant NP vRNAs by using minigenome assays, in which the NFLAG-NP protein was expressed together with PB1, PB2, and PA proteins from plasmids, and the amount of the luciferase reporter protein produced by a virus-like RNA was measured (data not shown). Because only the N49ATC-FLAG NP vRNA has most of the packaging signal sequence among the three recombinant NP vRNAs, these results suggest that the packaging signal is essential for the production of replication-competent virions containing the eight different vRNAs.
Fig 3.
Generation of virions with recombinant NP vRNAs. (A) Cell lysates of 18 plasmid (8 plasmids for vRNA expression and 10 plasmids for viral protein expression)-transfected cells (lanes 1 to 4), 17 plasmid (7 plasmids for vRNA expression except for the NP vRNA and 10 plasmids for viral protein expression)-transfected cells (lane 5), mock-transfected cells (lane 6), or one plasmid-transfected cells (lane 7) were subjected to Western blotting. Blots were probed with an anti-FLAG antibody, anti-WSN R309 serum, or a mouse anti-β-actin antibody. (B) MDCK cells infected with the viruses that were produced with each recombinant NP vRNA were stained with crystal violet. (C) The virus titers in the supernatants of the plasmid-transfected 293T cells were determined by use of plaque assays. Each panel represents two independent experiments that were performed in triplicate. The values are means ± the standard deviations from the three independent experiments. Statistical analysis was performed with the paired Student t test.
NP vRNA that lacks a packaging signal is incorporated into virions, but its packaging signal is necessary for the incorporation of the full set of vRNAs.
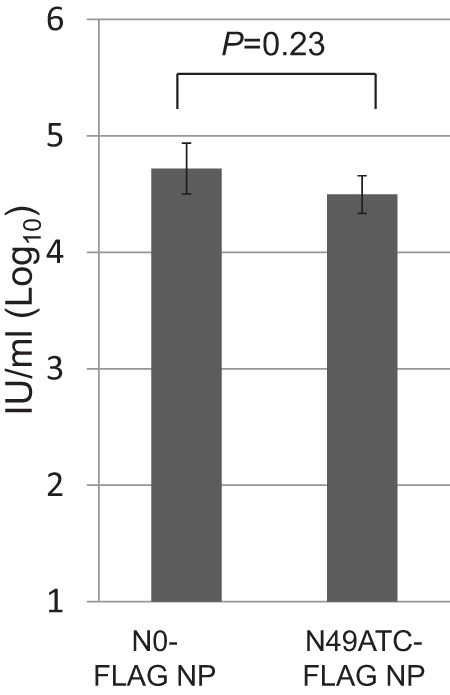
On the basis of the results shown in Fig. 3, we hypothesized that a large portion of the virions generated with N0-FLAG NP vRNA and N49ATCinv-FLAG NP vRNA (i.e., recombinant NP vRNAs lacking the complete packaging signal) would lack the complete set of eight different vRNAs, resulting in the formation of replication-deficient virions. To test this hypothesis, we attempted to generate viruses containing N0-FLAG NP vRNA, N49ATC-FLAG NP vRNA, or N49ATCinv-FLAG NP vRNA by reverse genetics. The supernatants containing virions were mixed with a helper WSN virus to provide NP in trans and inoculated to MDCK cells, which were then immunostained with an anti-FLAG antibody at 7 h postinfection. The FLAG-expressing cells were detected in MDCK cells incubated with the virus possessing N49ATC-FLAG NP vRNA, N0-FLAG NP vRNA, or N49ATCinv-FLAG NP vRNA (Fig. 4A). The number of FLAG-positive cells differed among the three viruses; those produced with N0-FLAG NP vRNA or N49ATCinv-FLAG NP vRNA being significantly lower than that of virus containing N49ATC-FLAG NP vRNA (Fig. 4B). However, the production efficiencies differed by only about 1 order of magnitude. Given that N0-FLAG NP vRNA and N49ATC-FLAG NP vRNA, which possess abnormal packaging signals, did not produce replication-competent virions containing eight different vRNAs (Fig. 3C) and that the packaging efficiency of the N0-FLAG NP vRNA was similar to that of the N49ATC-FLAG NP vRNA in the absence of the other 7 vRNAs (Fig. 5), these results suggest that a large portion of virions generated with N0-FLAG NP vRNA and N49ATCinv-FLAG NP vRNA likely lacked the complete set of eight vRNA segments. Thus, the packaging signal of NP vRNA is not essential for the efficient incorporation of NP vRNA; rather, it is important for the production of complete virions, that is, those possessing the complete eight set of vRNAs.
Fig 4.
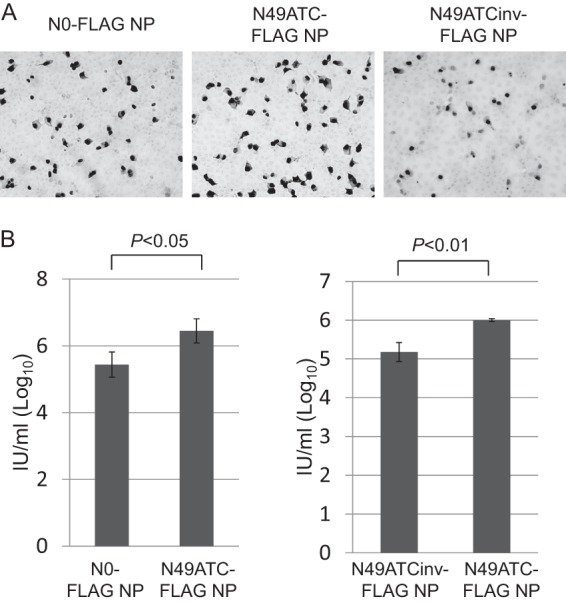
Detection of NFLAG-NP in virus-infected cells. (A) MDCK cells were infected with virions containing the recombinant NP vRNA. After a 7-h incubation, the FLAG epitope was detected by immunostaining with an anti-FLAG antibody. (B) Infection units (IUs), which represent the number of virions that can initiate infection and express vial proteins, were determined by detecting the number of cells that expressed NFLAG-NP. The values are means ± the standard deviations from three independent experiments. Statistical analysis was performed using the paired Student t test.
Fig 5.
Detection of NFLAG-NP in VLP-infected cells. MDCK cells were infected with VLPs, which were produced with a plasmid for the expression of the recombinant NP vRNA and 10 plasmids for the expression of the viral proteins, together with a helper virus. After a 7-h incubation, the FLAG epitope was detected by immunostaining with an anti-FLAG antibody. IUs were determined by detecting the number of cells that expressed NFLAG-NP. The values are means ± the standard deviations from three independent experiments. Statistical analysis was performed using the paired Student t test.
DISCUSSION
Although the selective packaging model for the influenza virus genome in which genome-packaging signals play a major role is now widely accepted, it has remained unclear how the packaging signals ensure that the eight different vRNAs are packaged. Here we used reverse genetics to show that the noncoding regions of the NP vRNA are sufficient for its incorporation into virions and that the packaging signal that includes both coding and noncoding elements of the NP vRNA is essential for the incorporation of a complete set of eight vRNAs into virions. We therefore propose that the noncoding regions of vRNAs serve as a virion incorporation signal and that the coding regions serve as a bundling signal that ensures the recruitment of the full set of vRNAs.
Consistent with previous reports (26, 27), the noncoding regions of NP vRNA were sufficient to pack the NP(0)GFP(0) vRNA into virus particles (Fig. 1). Interestingly, the terminal coding sequences adjacent to the noncoding regions did not increase the packaging efficiency of the NP(120)GFP(120) vRNA in the absence of the other vRNAs (Fig. 1), suggesting that the 3′ and 5′ noncoding regions per se are sufficient to signal the incorporation of that vRNA. In contrast, in the presence of the other seven vRNAs, the incorporation efficiency of the NP(120)GFP(120) vRNA was increased (Fig. 1), implying that the other vRNAs play a critical role in augmenting the packaging efficiency of the recombinant NP vRNA itself, possibly by direct or indirect interactions with the packaging signal of the recombinant NP vRNA (5, 6, 25).
How do the packaging signals ensure that all eight different vRNAs are incorporated? As shown in Fig. 3 and 4, N0-FLAG NP vRNA and N49ATCinve-FLAG NP vRNA did not support the production of replication-competent virions, although N0-FLAG NP vRNA and N49ATCinve-FLAG NP vRNA could be incorporated into virus particles. These findings suggest that the replication-deficient virions produced with N0-FLAG NP vRNA or N49ATCinve-FLAG NP vRNA lacked the complete set of eight vRNAs. Given that the packaging efficiency of the mutant NP vRNAs used here was similar in the absence of the other seven vRNAs (Fig. 5), it appears that the packaging signal of NP vRNA plays an important role in recruiting the other vRNAs into the virion during the genome-packaging process. Thus, it may be that the eight individual vRNAs mutually recruit the other vRNAs to ensure that the full complement of vRNAs is incorporated into the virions.
On the basis of our findings, we propose that the traditional packaging signal, which includes the terminal coding region and the noncoding regions at the 3′ and 5′ ends of each vRNA, can be divided into two separate signals: the incorporation signal and the bundling signal (Fig. 6). The incorporation signal, defined as the sequence required for the incorporation of the vRNA in which it resides, is located in the 3′ and 5′ noncoding regions. A previous study showed that a recombinant vRNA composed of the antisense-oriented chloramphenicol acetyltransferase gene flanked by the 3′ and 5′ noncoding regions (i.e., the recombinant vRNA possessed only the incorporation signal) was sufficient for the packaging of that vRNA (27). However, the virus lost the recombinant vRNA after several passages, indicating that this incorporation signal sequence is not sufficient for the segment to be maintained stably within the virions over multiple replication cycles (27). Another report supports this notion; when reporter vRNAs possessing the antisense-oriented green or yellow fluorescent protein gene flanked by the 3′ and 5′ noncoding regions were used for virus production in a reverse genetics system (i.e., the reporter vRNA possessed only the incorporation signal), 3 to 5% of the virus particles carried at least two copies of the reporter gene (28), suggesting that these vRNAs with only the incorporation signal were incorporated into virions in a somewhat nonspecific manner, where interactions between RNP and M1 may also be involved (29). Such nonspecific packaging of the vRNAs may allow virions to inadvertently carry more than eight vRNAs (30, 31).
Fig 6.
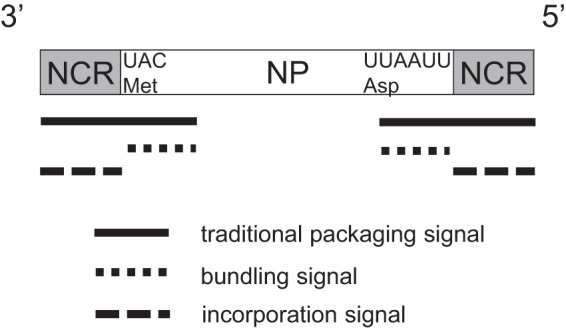
Schematic diagram of the NP vRNA. The NCR and CR of the NP vRNA are represented by gray and white boxes, respectively. The traditional packaging signal, the bundling signal, and the incorporation signal are represented by black, short dashed, and dashed bars, respectively.
The bundling signal, which is defined as the sequence that signals the incorporation of the other vRNAs, should correspond to the terminal coding regions of the vRNA. In the absence of the other vRNAs, the bundling signal is not involved in the efficient virion incorporation of the vRNA in which it resides (Fig. 1 and 5). However, in the presence of the other vRNAs, the bundling signal increases both the incorporation efficiency of the vRNA in which it resides (Fig. 1) and that of the other vRNAs (Fig. 1 and 3). This is likely because the bundling signal of one vRNA may interact with that of another vRNA (5, 6, 25), and vice versa, to mutually increase the incorporation efficiencies of the vRNAs. Previous studies have shown that a recombinant vRNA that possesses only the incorporation signal does not specifically compete with the other vRNAs in the genome-packaging process (8, 28), whereas a defective-interference (DI) vRNA, which typically contains the bundling signal, competes with its parental vRNA specifically (32, 33). Thus, it is possible that the bundling signal not only increases the incorporation efficiencies of vRNAs but also confers the selective mechanism upon the genome-packaging process.
The bundling signal appears to possess another important feature. Previous studies have shown that vRNA containing a reporter gene flanked by its 3′ and 5′ noncoding regions (i.e., only the strict packaging signal) is not maintained stably during multiple replication cycles (9, 27). However, a DI vRNA containing the 3′ and 5′ noncoding regions, as well as the additional coding sequences (i.e., the bundling signal), is stably maintained during virus replication (32, 33). These reports suggest that the bundling signal is required for the virus to stably maintain the vRNAs in a specific manner and therefore is responsible for the selectivity of vRNA packaging.
In conclusion, by using a reverse genetics system, we demonstrated that the traditional packaging signal consists of a virion incorporation signal and a bundling signal. This finding provides important insights into how influenza viruses ensure the integrity of their genome. Further studies are needed to better understanding the selective genome-packaging mechanism of influenza viruses, which could yield excellent targets for the development of anti-influenza drugs that inhibit the assemblage and incorporation of the viral genome.
ACKNOWLEDGMENTS
We thank Susan Watson for editing the manuscript.
This study was supported by grants-in-aid from the Ministry of Health, Labor, and Welfare of Japan, by ERATO (Japan Science and Technology Agency), by Public Health Service research grants from the National Institute of Allergy and Infectious Diseases, and by JSPS KAKENHI grant 21580372. T.N. was supported by a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science, by the Takeda Science Foundation, by the Senri Life Science Foundation, and by the Uehara Memorial Foundation.
Footnotes
Published ahead of print 8 August 2013
REFERENCES
- 1.Hutchinson EC, von Kirchbach JC, Gog JR, Digard P. 2010. Genome packaging in influenza A virus. J. Gen. Virol. 91:313–328 [DOI] [PubMed] [Google Scholar]
- 2.Noda T, Kawaoka Y. 2010. Structure of influenza virus ribonucleoprotein complexes and their packaging into virions. Rev. Med. Virol. 20:380–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oxford JS, Hockley DJ. 1998. Orthomyxoviridae. Perspect. Med. Virol. 3:213–232 [Google Scholar]
- 4.Noda T, Sagara H, Yen A, Takada A, Kida H, Cheng RH, Kawaoka Y. 2006. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439:490–492 [DOI] [PubMed] [Google Scholar]
- 5.Noda T, Sugita Y, Aoyama K, Hirase A, Kawakami E, Miyazawa A, Sagara H, Kawaoka Y. 2012. Three-dimensional analysis of ribonucleoprotein complexes in influenza A virus. Nat. Commun. 3:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fournier E, Moules V, Essere B, Paillart JC, Sirbat JD, Isel C, Cavalier A, Rolland JP, Thomas D, Lina B, Marquet R. 2012. A supramolecular assembly formed by influenza A virus genomic RNA segments. Nucleic Acids Res. 40:2197–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou YY, Vafabakhsh R, Doğanay S, Gao Q, Ha T, Palese P. 2012. One influenza virus particle packages eight unique viral RNAs as shown by FISH analysis. Proc. Natl. Acad. Sci. U. S. A. 109:9101–9106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagaki A, Goto H, Kakugawa S, Ozawa M, Kawaoka Y. 2012. Competitive incorporation of homologous gene segments of influenza A virus into virions. J. Virol. 86:10200–10202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii Y, Goto H, Watanabe T, Yoshida T, Kawaoka Y. 2003. Selective incorporation of influenza virus RNA segments into virions. Proc. Natl. Acad. Sci. U. S. A. 100:2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe T, Watanabe S, Noda T, Fujii Y, Kawaoka Y. 2003. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J. Virol. 77:10575–10583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii K, Fujii Y, Noda T, Muramoto Y, Watanabe T, Takada A, Goto H, Horimoto T, Kawaoka Y. 2005. Importance of both the coding and the segment-specific noncoding regions of the influenza A virus NS segment for its efficient incorporation into virions. J. Virol. 79:3766–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dos Santos Afonso E, Escriou N, Leclercq I, van der Werf S, Naffakh N. 2005. The generation of recombinant influenza A viruses expressing PB2 fusion protein requires the conservation of a packaging signal overlapping the coding and noncoding regions at the 5′ end of the PB2 segment. Virology 341:34–46 [DOI] [PubMed] [Google Scholar]
- 13.Liang Y, Hong Y, Parslow TG. 2005. cis-Acting packaging signals in the influenza virus PB1, PB2, and PA genomic RNA segments. J. Virol. 79:10348–10355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muramoto Y, Takada A, Fujii K, Noda T, Iwatsuki-Horimoto K, Watanabe S, Horimoto T, Kida H, Kawaoka Y. 2006. Hierarchy among viral RNA (vRNA) segments in their role in vRNA incorporation into influenza A virions. J. Virol. 80:2318–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozawa M, Fujii K, Muramoto Y, Yamada S, Yamayoshi S, Takada A, Horimoto T, Kawaoka Y. 2007. Contributions of two nuclear localization signals of influenza A virus nucleoprotein to viral replication. J. Virol. 81:30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozawa M, Maeda J, Iwatsuki-Horimoto K, Watanabe S, Goto H, Horimoto T, Kawaoka Y. 2009. Nucleotide sequence requirements at the 5′ end of the influenza A virus M RNA segment for efficient virus replication. J. Virol. 83:3384–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noda T, Kawaoka Y. 2012. Packaging of influenza virus genome: robustness of selection. Proc. Natl. Acad. Sci. U. S. A. 109:8797–8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh GA, Hatami R, Palese P. 2007. Specific residues of the influenza A virus hemagglutinin viral RNA are important for efficient packaging into budding virions. J. Virol. 81:9727–9736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gog JR, Afonso Edos S, Dalton RM, Leclercq I, Tiley L, Elton D, von Kirchbach JC, Naffakh N, Escriou N, Digard P. 2007. Codon conservation in the influenza A virus genome defines RNA packaging signals. Nucleic Acids Res. 35:1897–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang Y, Huang T, Ly H, Parslow TG. 2008. Mutational analyses of packaging signals in influenza virus PA, PB1, and PB2 genomic RNA segments. J. Virol. 82:229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh GA, Rabadan R, Levine AJ, Palese P. 2008. Highly conserved regions of influenza A virus polymerase gene segments are critical for efficient viral RNA packaging. J. Virol. 82:2295–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchinson EC, Curran MD, Read EK, Gog JR, Digard P. 2008. Mutational analysis of cis-acting RNA signals in segment 7 of influenza A virus. J. Virol. 82:11869–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchinson EC, Wise HM, Kudryavtseva K, Curran MD, Digard P. 2009. Characterization of influenza A viruses with mutations in segment 5 packaging signals. Vaccine 27:6270–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujii K, Ozawa M, Iwatsuki-Horimoto K, Horimoto T, Kawaoka Y. 2009. The incorporation of influenza A virus genome segments dose not absolutely require wild-type sequences. J. Gen. Virol. 90:1734–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fournier E, Moules V, Essere B, Paillart JC, Sirbat JD, Cavalier A, Rolland JP, Thomas D, Lina B, Isel C, Marquet R. 2012. Interaction network linking the human H3N2 influenza A virus genomic RNA segments. Vaccine 30:7359–7367 [DOI] [PubMed] [Google Scholar]
- 26.Neumann G, Watanabe T, Kawaoka Y. 2000. Plasmid-driven formation of influenza virus-like particles. J. Virol. 74:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luytjes W, Krystal M, Enami M, Parvin JD, Palese P. 1989. Amplification, expression, and packaging of a foreign gene by influenza virus. Cell 59:1107–1113 [DOI] [PubMed] [Google Scholar]
- 28.Bancroft CT, Parslow TG. 2002. Evidence for segment-nonspecific packaging of the influenza A virus genome. J. Virol. 76:7133–7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noton SL, Medcalf E, Fisher D, Mullin AE, Elton D, Digard P. 2007. Identification of the domains of the influenza A virus M1 matrix protein required for NP binding, oligomerization, and incorporation into virions. J. Gen. Virol. 88:2280–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enami M, Sharma G, Benham C, Palese P. 1991. An influenza virus containing nine different RNA segments. Virology 185:291–298 [DOI] [PubMed] [Google Scholar]
- 31.Scholtissek C, Rohde W, Harms E, Rott R, Orlich M, Boschek CB. 1978. A possible partial heterozygote of an influenza A virus. Virology 89:506–516 [DOI] [PubMed] [Google Scholar]
- 32.Duhaut SD, Dimmock NJ. 1996. Defective RNAs inhibit the assembly of influenza virus genome segments in a segment-specific manner. Virology 216:326–337 [DOI] [PubMed] [Google Scholar]
- 33.Odagiri T, Tashiro M. 1997. Segment-specific noncoding sequences of the influenza virus genome RNA are involved in the specific competition between defective interfering RNA and its progenitor RNA segment at the virion assembly step. J. Virol. 71:2138–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]