Fig 2.
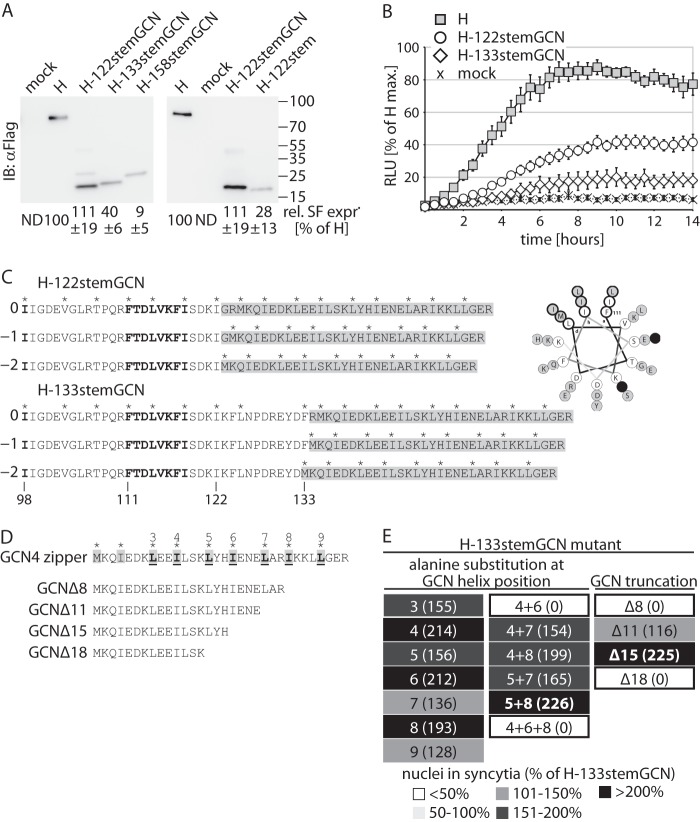
GCN4 zipper domain-stabilized H-stems are intracellular transport and F triggering competent. (A) Surface expression (SF expr.) analysis of the H-stem constructs. Immunoblots (IB) after surface biotinylation were decorated with specific antibodies directed against a Flag epitope tag added to the cytosolic tail of all H constructs. Control cells were transfected with vector DNA only (mock). Numbers represent densitometric quantification relative (Rel.) to standard MeV H. Averages of the results of three independent experiments ± standard errors of the means (SEM) are shown. ND, not determined. (B) Real-time content mixing assay to quantify the kinetic of productive F triggering. Effector cells coexpressing an H variant, F, and one DSP subunit were mixed with target cells expressing the complementary DSP component, and relative luciferase units (RLU) were measured continuously over a 24-h period. Results were normalized for peak values observed with standard H and represent averages of the results of three independent experiments ± SEM. (C) (Left panel) Schematic of the relative helical wheel variations introduced between the H-stem and GCN4 zipper domain. Asterisks above the sequences denote hydrophobic positions in the helix repeat pattern. The numbers “0,” “−1,” and “−2” refer to the three relative helix positions at the junction. Residues in bold highlight the previously identified F-triggering and F interaction positions. Gray shading marks the GCN4-derived tetramerization tags. (Right panel) Helical wheel projection illustrating the pattern variation at the H-stem/GCN zipper intersection by the example of H-122stemGCN. The projection commences at H residue 111. Hydrophobic helix positions (a, d, h) are highlighted. The transition from the predicted 11-mer repeat of the H stalk to the 7-mer repeat of the GCN zipper disrupts the helical pattern (black filled circles). H-stem residues are shown in open circles and GCN residues in gray-shaded circles. (D) Mutagenesis and truncation of the GCN4 zipper domain. Numbered residues (bold, underlined) were mutagenized. Truncations are calculated from the carboxy terminus. (E) GCN-5A8A double mutants and GCN-Δ15 truncations showed the highest F-triggering activities. Values in parentheses represent the extent of fusion relative to that observed for the starting H-133stemGCN construct. Selected modifications with the highest activities are shown in bold.