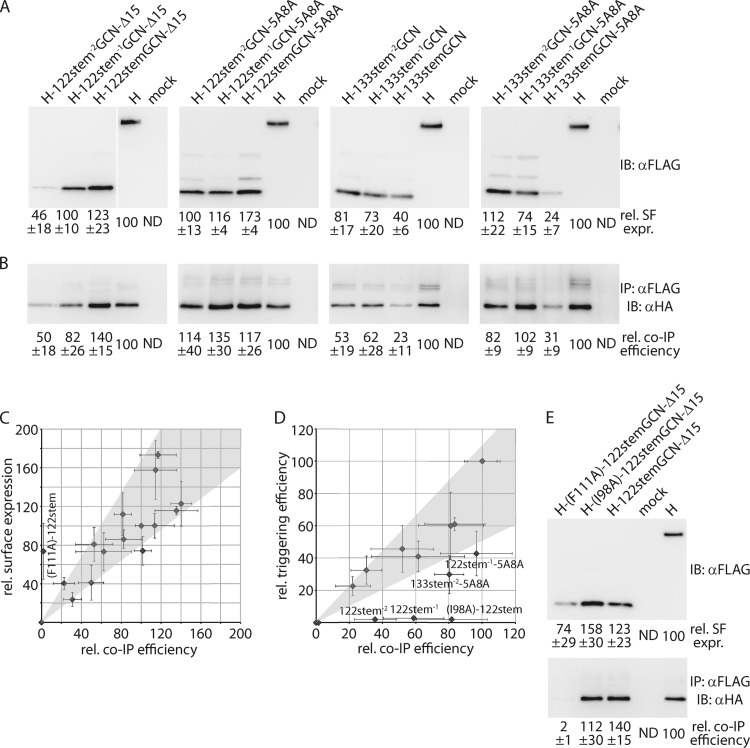
Fig 4.
Stabilized H-stem variants are surface expressed and F interaction competent. (A) Surface expression of two complete series of each stem variant, associated with the highest triggering activities. (B) Coimmunoprecipitation (IP) of cross-linked, cell surface-exposed H-F complexes. Immunoblots (IB) were developed with specific antibodies directed against the HA epitope tag, affixed to the cytosolic tail of the F protein. For panels A and B, numbers show densitometry averages ± SEM relative to standard H of the results of three independent experiments. (C) Surface levels of individual H-stems tested as described for panel A directly correlate with co-IP efficiency. Average values obtained as described for panels A and B are plotted. The H-(F122A)-122stemGCN-Δ15 mutant included for control is F binding defective. (D) Several H-stem constructs (specified) are overproportionally F triggering defective. Average kinetic fusion data of the 14-h time points (Fig. 3A to C) are plotted as a function of relative co-IP efficiencies. H-stem122 and H-stem133 series co-IP values were normalized separately for H-122stemGCN-Δ15 and H-133stemGCN-5A8A, respectively. The H-(I98A)-122stemGCN-Δ15 mutant included for control is F triggering defective. Gray areas in panels C and D represent the linear regression line of the data sets ± 1 standard deviation. (E) Surface biotinylation and H-F coprecipitation of the H-122stemGCN-Δ15 (I98A) and (F111A) mutants used as described for panels C and D for control. Numbers represent densitometry averages of the results of three independent experiments ± SEM.