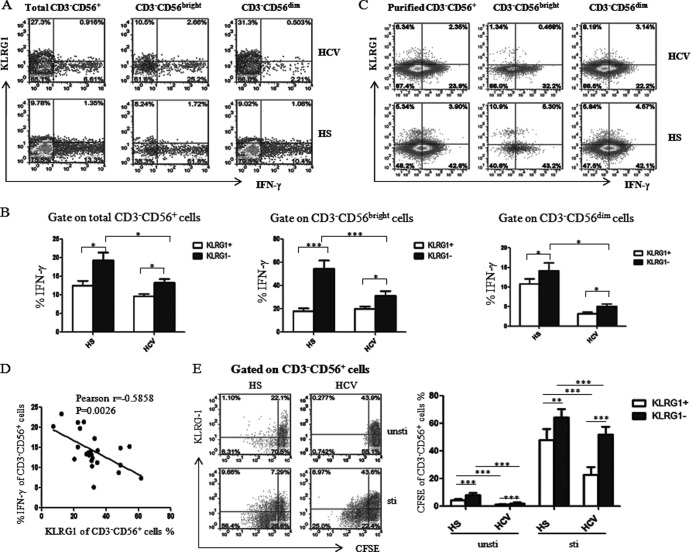
Fig 3.
KLRG1 expression is inversely associated with the low levels of IFN-γ production by NK cells in HCV infection. (A) PBMCs from chronically HCV-infected patients and HS were stimulated with rhIL-12 (10 ng/ml) for 18 h and incubated for the last 4 h with brefeldin A (10 μg/ml) to halt cytokine secretion. The cells were immunostained with PE-CD3, PerCP-CD56, APC–IFN-γ, and Alexa Fluor 488-KLRG1, and the IFN-γ production by KLRG1+ and KLRG1− NK cells was analyzed by flow cytometry. Representative dot plots for IFN-γ production by KLRG1+ and KLRG1− NK cells, including total CD3− CD56+, CD3− CD56bright, and CD3− CD56dim subsets from an HCV-infected subject and HS. (B) The average percentage of IFN-γ production by the KLRG1+ versus KLRG1− fraction of total CD3− CD56+ NK cells, or in the CD3− CD56bright and CD3− CD56dim subsets. Results are expressed as means ± SD of the percentages of IFN-γ production by NK cells from 24 HCV-infected patients versus 8 HS. *, P < 0.05; ***, P < 0.001. (C) Representative dot plots for KLRG1 expression and IFN-γ production by negatively purified NKs, including total CD3− CD56+ and CD3− CD56bright or CD3− CD56dim subsets from an HCV-infected subject and HS. The percentage of cells in the gated area is shown in the dot plots. (D) Relationship between KLRG1 expression and IFN-γ production by CD3− CD56+ NK cells from 24 HCV-infected patients based on Pearson correlation analysis (r = −0.585; **, P < 0.01). E) KLRG1+ and KLRG1− NK cell proliferation levels were assessed in a CFSE assay. PBMCs were labeled with CFSE and cultured with or without rhIL-12 and rhIL-2 for 6 days. The cells were immunostained with PE-CD3, PerCP-CD56, and Alexa Fluor 488-KLRG1 and analyzed by flow cytometry as described in Materials and Methods. Representative dot plots (left panel) and summary data (right panel; mean ± SD) of CFSE dilution in the KLRG1+ and KLRG1− NK cells from 8 HCV patients versus 8 HS, with and without ex vivo stimulation, are shown. ***, P < 0.001.