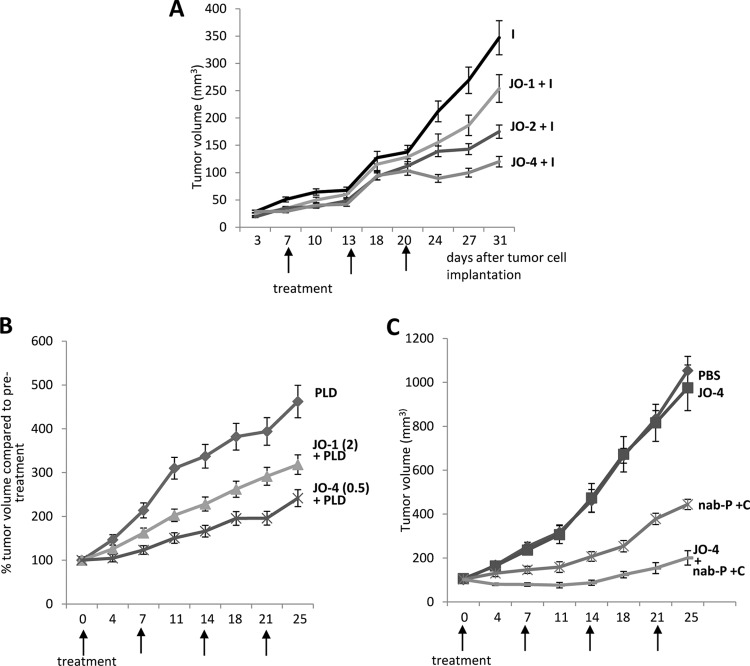
Fig 10.
Combination of affinity-enhanced JO-1 versions with chemotherapy. (A) Enhancement of irinotecan (I) therapy. The experimental settings were the same as those described for Fig. 5B. The differences in the groups JO-1 plus I versus JO-2 plus I and JO-2 plus I versus JO-4 plus I were significant from day 20 on. n = 5. (B) JO-4 enhances PLD therapy in an ovarian cancer model at a lower dose than JO-1. Mammary fat pad tumors were established from primary ovarian cancer Ovc316 cells. Treatment was started when tumors reached a volume of 100 mm3. Mice were injected intravenously with 2 mg/kg JO-1 or with 0.5 mg/kg JO-4, followed by an intravenous injection of PLD (1 mg/kg) 1 h later. Treatment was repeated weekly. (C) JO-4 enhances therapy in poor-prognosis triple-negative breast cancer (TNBC). A total of 4 × 106 TNBC MDA-MB-231 cells were injected into the mammary fat pad of CB17 SCID/beige mice. JO-4 (2 mg/kg) was intravenously injected 1 h before the application of cetuximab (C) (10 mg/kg, intraperitoneal) and nab-paclitaxel (nab-P) (5 mg/kg, intravenous). Treatment was given weekly. n = 10. P < 0.01 at day 25 for nab-P plus C versus JO-4 plus nab-P plus C.