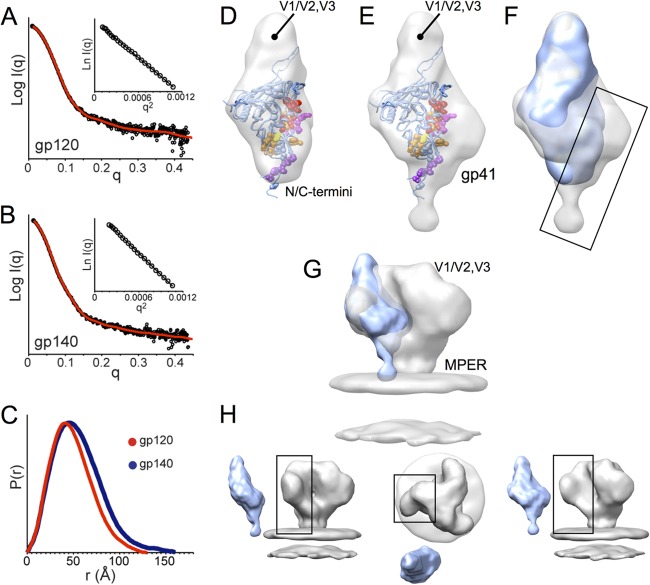
Fig 6.
SAXS of monomeric gp120 and gp140 SF162 suggests gp41 is positioned alongside the gp120 inner domain. (A and B) Experimental SAXS patterns (black) measured for gp120 (A) and gp140 (B) monomers compared to fits obtained from ab initio reconstructions using DAMMIN (red) (47). Insets show the linearity of the Guinier plots. (C) Distance distribution [P(r)] plots for gp120 (red) and gp140 (blue) obtained from GNOM (47). (D and E) Shape reconstructions for monomeric gp120 (D) and monomeric gp140 (E) are shown with the structural model (PDB 3JWD and 2B4C to show approximate locations for N/C-terminal extensions and V3) docked into the SAXS envelope; gp120 core residues that have been identified as playing a role in association with gp41 are shown in space-filling balls and colored as C-terminal extenions (purple), β-sheet “platform” (yellow), layer 1 (magenta), and layer 2 (red) (60). (F) Comparison of SAXS reconstructions for gp120 (blue) and gp140 monomers (gray) reveals additional density in the gp140 monomer localized around the putative gp41 interactive face of gp120 (box). (G and H) Comparison of a gp140 monomer SAXS model with cryo-EM reconstruction for the Env trimer (gray, EMD-5019 [9]) shows good agreement, suggesting that the protomer retains large-scale organization similar to what exists in the membrane-presented Env trimer.