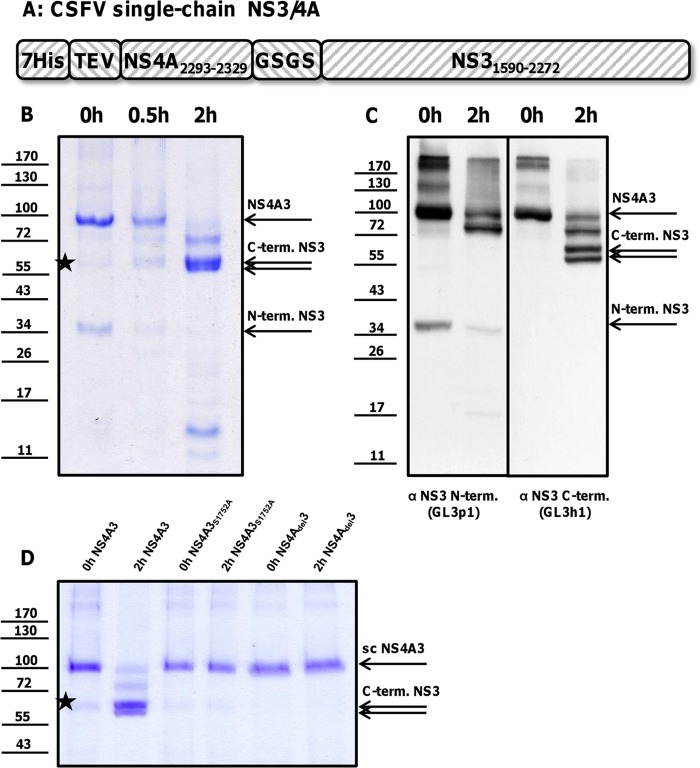
Fig 3.
(A) Internal cleavage of a CSFV NS4A3 construct. (B) NS4A3 immediately after purification by IMAC (0 h NS4A3) or after incubation for 0.5 h or 2 h at 37°C (0.5 h or 2 h NS4A3) was solved by SDS-PAGE and visualized by Coomassie staining. (C) Degradation products were specified using antibodies against the N and C termini of NS3 (the applied antibodies are indicated below the figure panel). The dominant protein bands were attributed to NS3 (75 kDa), two C-terminal fragments (58 and 55 kDa), and one short-lived N-terminal fragment (35 kDa). To inactivate the NS4A3 protease, a part of the NS4A cofactor peptide was deleted (NS4Adel3) or the essential Ser1752 was mutated (NS4A3S1752A). Inactivation of the NS4A3 protease stabilized the construct (shown in panel D). The positions of NS4A3 and processing products are indicated at the right. A weak 58-to-60-kDa band visible in all preparations (marked with stars in panels B and D) is most likely a copurified bacterial protein that is not detected by NS3-specific antibodies (C). Molecular mass standards (kDa) are shown on the left.