Abstract
The human herpesvirus 6 (HHV-6) envelope glycoprotein gH/gL/gQ1/gQ2 complex associates with host cell CD46 as its cellular receptor. Although gB has been suggested to be involved in HHV-6 infection, its function in membrane fusion has remained unclear. Here, we have developed an HHV-6A (strain GS)and HHV-6B (strain Z29) virus-free cell-to-cell fusion assay and demonstrate that gB and the gH/gL/gQ1/gQ2 complex are the minimum components required for membrane fusion by HHV-6.
TEXT
Human herpesvirus 6 (HHV-6), betaherpesvirus subfamily (1), includes two species, A (HHV-6A) and B (HHV-6B) (2–4). HHV-6B mainly infects immune cells, such as CD4+ T-lymphocytes, monocytes, and dendritic cells, and also causes exanthema subitum during primary infection in children (5). HHV-6B can reactivate from latency in immunocompromised patients and cause pneumonitis, hepatitis, and encephalitis (6, 7). However, the molecular basis of HHV-6A pathogenicity is unclear.
The association of several viral glycoproteins with their respective cellular receptors induces virus envelope-cell membrane fusion during viral entry. It has been reported that HHV-6 gH/gL forms a complex with gQ1 and gQ2 and that this complex binds to CD46, which has been reported to function as a cellular receptor for HHV-6 (8–11). gB and a gH/gL complex are conserved in all herpesviruses and thought to play a pivotal role in membrane fusion and herpesvirus infection (12–17). Studies of gBs and gHs of other herpesviruses have elucidated the molecular mechanisms of virus envelope-cell membrane fusion (18–21). Although some antibodies against HHV-6 gB have been reported to block HHV-6B infection (22, 23), the function of HHV-6 gB during viral infection remains unclear.
To identify the requirement of HHV-6 glycoproteins for virus-induced membrane fusion during the virus infection, each of the glycoproteins was amplified and expressed from HHV-6B (Z29). Briefly, the genomic sequences of gH, gL, gO, gQ1, and gQ2 were amplified from total DNA of HHV-6B-infected Molt3 cells (Riken BRC, Tsukuba, Japan) and cloned into pCAGGS-MCS expression vector (24). For detection purposes, the FLAG epitope was inserted in frame at the N termini of gO and gQ2 genes. The full-length gB gene containing a promoter and poly(A) tail sequences was amplified by recombinant PCR using plasmids containing partial gB sequences (nucleotides [nt] +1 to +1718 and +1713 to +2493). The purified PCR product was used for transient transfection of 293T cells. Expression of transfected genes was analyzed by flow cytometry. gB and the gH/gL complex were detected on the cell surface using anti-gB monoclonal antibody (MAb) and gHA2 antibody, respectively (Fig. 1A) (25). Cells transfected with plasmid encoding gQ1 or N-terminal FLAG-tagged gQ2 expressed the corresponding proteins. gQ1 and gQ2 were detected intracellularly but not on the cell surface, although they were detected on the surface of cells cotransfected with gH and gL. N-terminal FLAG-tagged gO was also expressed only on the surface of cells cotransfected with gH and gL (data not shown). The level of gB expression on HHV-6B-infected cells was higher than on gB-transfected cells. However, the levels of gH and gQ1 expression on transfected cells were higher than on infected cells (Fig. 1A and B).
Fig 1.
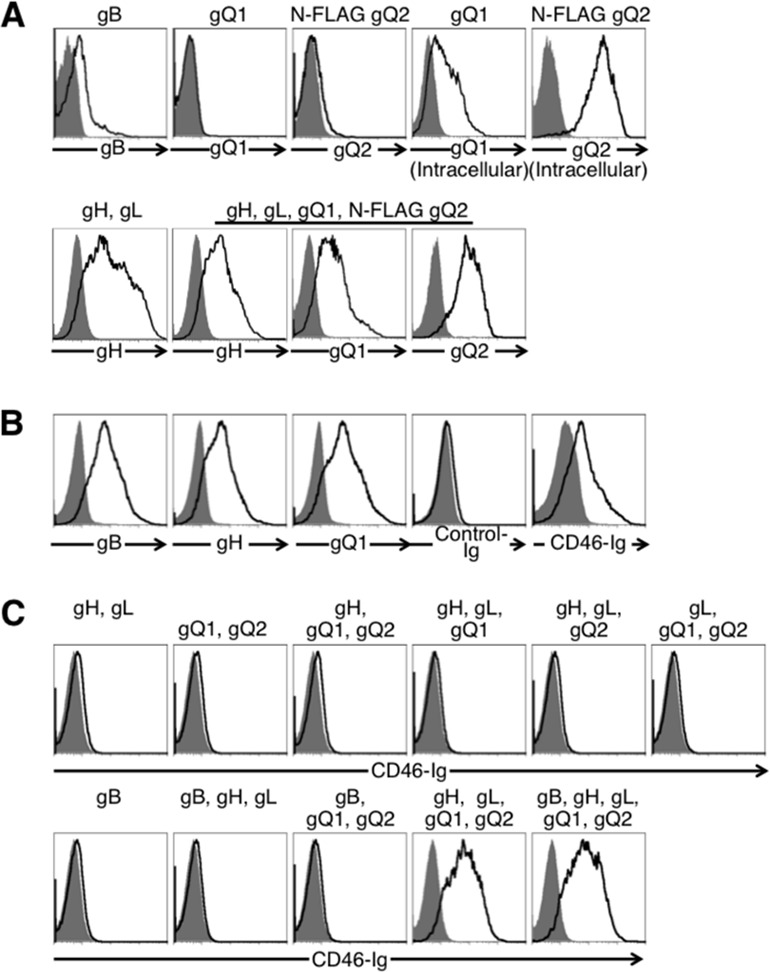
Flow cytometric analyses of cell surface expression of viral glycoproteins in cells transfected with plasmids expressing the glycoproteins. The transfected glycoprotein(s) is shown at the top of each figure panel. gQ2 was FLAG tagged. (A) Expression of HHV-6B glycoprotein(s) in 293T cells transfected with plasmids expressing HHV-6B glycoprotein(s) (black lines) or mock transfected (gray-shaded areas). Cells were stained with anti-gB (H-AR-2; Bioworld Consulting Laboratories), anti-gH, anti-gQ1 (2D6; NIH, AIDS Reagent Program), or FLAG (L5; Biolegend) MAb followed by staining with anti-mouse IgG antibody. (B) Cell surface expresson of HHV-6B glycoproteins in virus-infected cells and association of CD46 with HHV-6B-infected cells. HHV-6B-infected (black lines) or mock-infected (gray-shaded areas) Molt-3 cells were stained with anti-gB, anti-gH, or anti-gQ1 MAb followed by staining with anti-mouse IgG antibody and either CD46-Ig or control Ig (VZV gB-Ig) followed by staining with anti-human IgG Fc portion antibody. (C) Association of CD46 with HHV-6B glycoproteins. 293T cells that were transfected with plasmids expressing HHV-6B glycoprotein(s) (black lines) or mock transfected (gray-shaded areas) were stained with CD46-Ig.
We then generated a flow cytometry analysis that used CD46-Ig fusion protein to analyze HHV-6B glycoproteins that bind to CD46 (26). CD46-Ig specifically associated with HHV-6B-infected Molt-3 cells but not mock-infected cells (Fig. 1B). The 293T cells which were transfected with HHV-6 glycoprotein(s) and stained with CD46-Ig showed that CD46-Ig did not bind to cells expressing gH and gL, gB alone, or gH, gL, and gB but did bind to cells transfected with gH, gL, gQ1, and gQ2 (Fig. 1C). Expression of gB did not affect CD46-Ig binding to cells expressing gH, gL, gQ1, and gQ2. These results suggested that CD46 associated with a gH/gL/gQ1/gQ2 complex on the cell surface.
To identify HHV-6 glycoproteins that mediate membrane fusion, we developed a HHV-6 virus-free cell-to-cell fusion assay. 293T effector cells were cotransfected with the plasmids expressing HHV-6B glycoproteins and a plasmid expressing DsRed or were mock transfected. 293T target cells were cotransfected with plasmid expressing CD46 and green fluorescent protein (GFP) (Fig. 2A). Effector cells were cocultured with target cells 24 h after transfection. After coculture for 72 h, the cells were analyzed by fluorescence microscopy. As shown in Fig. 2B, yellow, giant, fused cells were observed when effector cells were cotransfected with plasmids expressing HHV-6B gB, gH, gL, gQ1, and gQ2 and cocultured with CD46-transfected target cells. However, no fused cells were found in the absence of gB.
Fig 2.
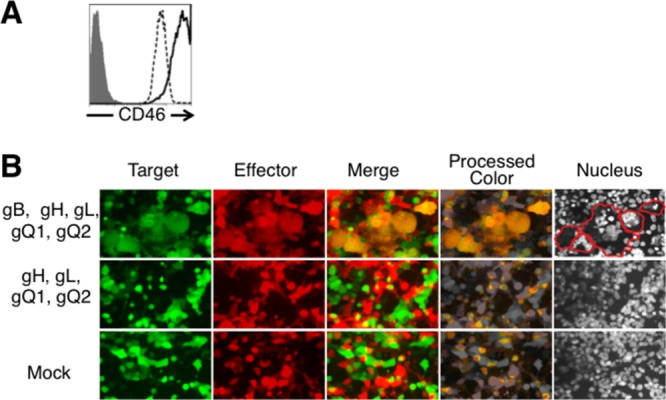
Fluorescence microscopy of fusion of 293T effector and target cells. (A) To quantify CD46 expression on the surface of 293T cells, 293T cells were stained with anti-CD46 MAb (J4.48; Coulter) (dotted line) or with isotype control antibody (gray-shaded area), and CD46-transfected 293T cells were stained with anti-CD46 MAb (solid line) and analyzed by flow cytometry. (B) 293T effector cells were transfected with plasmids expressing HHV-6B glycoproteins or mock transfected with a plasmid expressing DsRed. 293T target cells were transfected with a plasmid expressing CD46 and a plasmid expressing GFP. After 72 h coculture, cells were analyzed by fluorescence microscopy. Cell nuclei were stained with Hoechst 33258 fluorescence dye; blue fluorescence from nuclei appears gray. Fused cells are delineated by red lines.
To quantify fusion efficiency, a dual-luciferase reporter assay was used as previously reported (15). 293T effector cells were cotransfected with plasmid expressing HHV-6B glycoproteins, T7 polymerase (pCAGT7), and Renilla luciferase (as an internal control) and cocultured with 293T target cells transfected with CD46 and T7 promoter-driven firefly luciferase (pT7EMCluc) for 72 h. Firefly and Renilla luciferase activities were then measured, and fusion efficiency was calculated as described in the Fig. 3 legend. The fusion efficiency of varicella-zoster virus (VZV) envelope glycoproteins was measured as a control (15). Cell-to-cell fusion was 10.2-fold more efficient with gB-, gH-, gL-, gQ1-, and gQ2-transfected effector cells than with mock-transfected effector cells (Fig. 3B). In the absence of gB, gH, gL, gQ1, or gQ2, no significant fusion activity was observed. gO of human cytomegalovirus (HCMV) and HHV-6 have been suggested to form a complex with gH and gL, with the complex being involved in HCMV entry (9, 27). However, transfection with HHV-6B gO did not affect cell-to-cell fusion induced by HHV-6B gB, gH, gL, gQ1, and gQ2 (data not shown). This is in agreement with the previous report that gO is not essential for HCMV cell-to-cell fusion (27). CD46-Ig also bound to HHV-6A (strain GS) gH, gL, gQ1, and gQ2 transfectants (data not shown), and cell-to-cell fusion was observed using HHV-6A envelope glycoproteins (Fig. 3A) (28). Furthermore, cell-to-cell fusion using either HHV-6A or -6B glycoproteins was inhibited by both anti-CD46 and anti-HHV-6A gB MAbs (clone 87-y-13), similar to reports in which syncytium formation by HHV-6A was abrogated by these MAbs (Fig. 4) (29, 30). These results suggested that both HHV-6A and HHV-6B require gB, gH, gL, gQ1, and gQ2 for cell-to-cell fusion.
Fig 3.
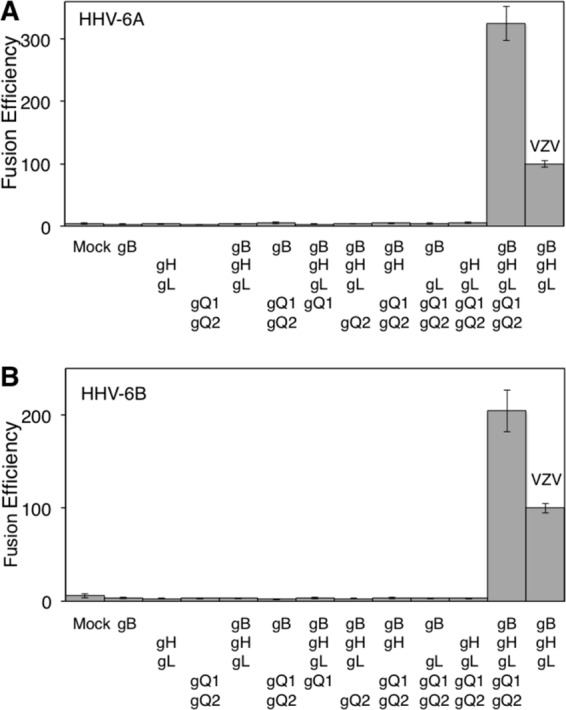
Quantification of cell-to-cell fusion mediated by HHV-6 glycoproteins. (A) 293T effector cells transfected with plasmids expressing HHV-6A glycoproteins, T7 polymerase, and Renilla luciferase were cocultured with 293T target cells transfected with plasmids expressing CD46 and firefly luciferase. After 72 h coculture, both luciferase signals were measured. The relative fusion efficiency was calculated as follows: [(HHV-6 firefly luciferase activity/HHV-6 Renilla luciferase activity) × 100]/(VZV firefly luciferase activity/VZV Renilla luciferase activity). (B) Quantification of cell-to-cell fusion efficiency mediated by HHV-6B glycoproteins was performed as described for HHV-6A in the panel A legend. Error bars show the means ± standard deviations (SD) of the results determined with quadruplicated samples. Data are representative of at least three independent experiments.
Fig 4.
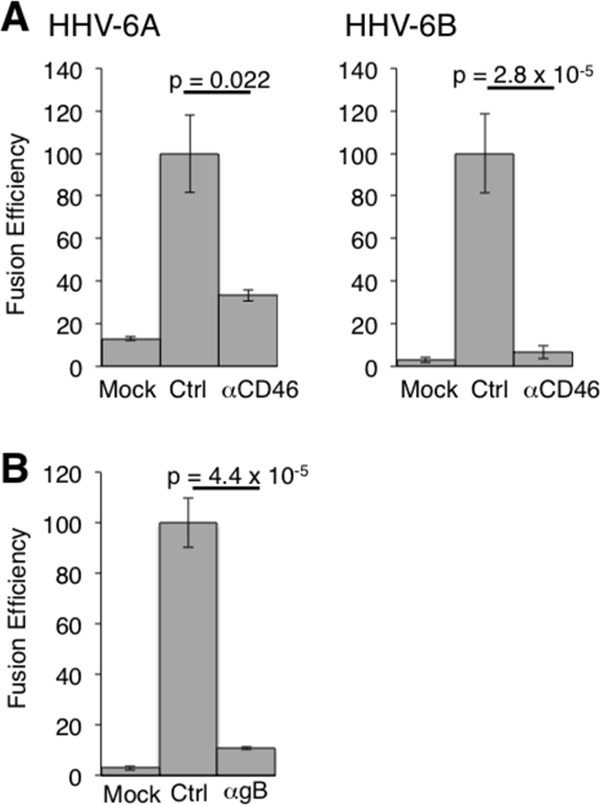
Effect of anti-CD46 and anti-gB MAbs on HHV-6-glycoprotein-mediated cell-to-cell fusion. (A) Cell-to-cell fusion efficiency mediated by HHV-6A and HHV-6B glycoproteins was measured in the presence of anti-CD46 MAb (M75), in the absence of anti-CD46 MAb (Ctrl), and in mock-transfected cells as described in the Fig. 3 legend. Fusion efficiency was calculated as follows: [(firefly luciferase activity/Renilla luciferase activity) × 100]/[(firefly luciferase activity/Renilla luciferase activity) in control cells]. (B) Cell-to-cell fusion efficiency mediated by HHV-6A glycoproteins was measured in the presence of anti-HHV-6A gB MAb (clone 87-y-13) and in the absence of anti-gB MAb (Ctrl) and in mock-transfected cells as described in the panel A legend. Error bars show the means ± SD of the results determined with quadruplicated samples. The statistical difference was determined by the Student's t test. A difference with P < 0.05 was considered statistically significant. Data are representative of at least three independent experiments.
Cell-to-cell fusion assays were also done in trans; i.e., some cells were transfected only with plasmid(s) gB, gH/gL, and/or gQ1/gQ2 and other cells were transfected with plasmids expressing all the other glycoproteins. Little cell-to-cell fusion was observed in in trans fusion assays (data not shown). These results suggested that cis expression of HHV-6 gB, gH, gL, gQ1, and gQ2 is required for cell-to-cell fusion, unlike that of herpes simplex virus (HSV) and HCMV, in which all the envelope glycoproteins do not need to be expressed on the same cell (17, 31).
This is the first report showing that the HHV-6A and HHV-6B envelope glycoproteins gB, gH, gL, gQ1, and gQ2 are required for cell-to-cell fusion. Herpesviruses enter via two different pathways: (i) direct fusion of the viral envelope with the host cell membrane or (ii) endocytosis followed by fusion between the viral envelope and endosomal membranes (32). Since membrane fusion is needed for herpesvirus entry, our results are consistent with previous reports that anti-gB, -gH, and -gQ1 antibodies block HHV-6 infection (22–24, 33–36). Moreover, our results are also supported by an earlier report that gB and gH are required for polykaryocyte formation after virus infection of permissive cells in cell culture (29). Considering that gBs and gHs of other herpesviruses associate with their respective cellular receptors during viral entry and cell-to-cell fusion (15, 26, 37–41), HHV-6 gB may also mediate viral entry and cell-to-cell fusion by interaction with cellular receptors that are currently unknown in addition to the binding of the gH, gL, gQ1, and gQ2 complex to its receptor CD46. The virus-free HHV-6 fusion assay system developed in this study should help elucidate the HHV-6 entry mechanism.
ACKNOWLEDGMENTS
We thank M. Mastumoto and K. Shida for technical help, Y. Mori (Kobe University) for providing HHV-6A (GS)-infected cells and anti-gH and anti-gB (clone 87-y-13) MAbs, and Y. Matsuura (Osaka University) for providing pCAGT7 and pT7EMCluc.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (T. Suenaga and H.A.) and in part by grants from the Takeda Science Foundation (T. Suenaga and H.A.).
Footnotes
Published ahead of print 24 July 2013
REFERENCES
- 1.Roizmann B, Desrosiers RC, Fleckenstein B, Lopez C, Minson AC, Studdert MJ. 1992. The family Herpesviridae: an update. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses. Arch. Virol. 123:425–449 [DOI] [PubMed] [Google Scholar]
- 2.Aubin JT, Collandre H, Candotti D, Ingrand D, Rouzioux C, Burgard M, Richard S, Huraux JM, Agut H. 1991. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J. Clin. Microbiol. 29:367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campadelli-Fiume G, Guerrini S, Liu X, Foa-Tomasi L. 1993. Monoclonal antibodies to glycoprotein B differentiate human herpesvirus 6 into two clusters, variants A and B. J. Gen. Virol. 74(Pt 10):2257–2262 [DOI] [PubMed] [Google Scholar]
- 4.Wyatt LS, Balachandran N, Frenkel N. 1990. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J. Infect. Dis. 162:852–857 [DOI] [PubMed] [Google Scholar]
- 5.Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065–1067 [DOI] [PubMed] [Google Scholar]
- 6.Asano Y, Yoshikawa T, Suga S, Yazaki T, Kondo K, Yamanishi K. 1990. Fatal fulminant hepatitis in an infant with human herpesvirus-6 infection. Lancet 335:862–863 [DOI] [PubMed] [Google Scholar]
- 7.Clark DA. 2002. Human herpesvirus 6 and human herpesvirus 7: emerging pathogens in transplant patients. Int. J. Hematol. 76(Suppl 2):246–252 [DOI] [PubMed] [Google Scholar]
- 8.Maeki T, Mori Y. 2012. Features of human herpesvirus-6A and -6B entry. Adv. Virol. 2012:384069. 10.1155/2012/384069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori Y, Akkapaiboon P, Yonemoto S, Koike M, Takemoto M, Sadaoka T, Sasamoto Y, Konishi S, Uchiyama Y, Yamanishi K. 2004. Discovery of a second form of tripartite complex containing gH-gL of human herpesvirus 6 and observations on CD46. J. Virol. 78:4609–4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori Y, Yang X, Akkapaiboon P, Okuno T, Yamanishi K. 2003. Human herpesvirus 6 variant A glycoprotein H-glycoprotein L-glycoprotein Q complex associates with human CD46. J. Virol. 77:4992–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817–827 [DOI] [PubMed] [Google Scholar]
- 12.Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. 2009. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc. Natl. Acad. Sci. U. S. A. 106:20464–20469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haan KM, Lee SK, Longnecker R. 2001. Different functional domains in the cytoplasmic tail of glycoprotein B are involved in Epstein-Barr virus-induced membrane fusion. Virology 290:106–114 [DOI] [PubMed] [Google Scholar]
- 14.Pertel PE. 2002. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J. Virol. 76:4390–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. 2010. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. U. S. A. 107:866–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner A, Bruun B, Minson T, Browne H. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanarsdall AL, Ryckman BJ, Chase MC, Johnson DC. 2008. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J. Virol. 82:11837–11850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat. Struct. Mol. Biol. 17:882–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220 [DOI] [PubMed] [Google Scholar]
- 21.Matsuura H, Kirschner AN, Longnecker R, Jardetzky TS. 2010. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proc. Natl. Acad. Sci. U. S. A. 107:22641–22646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foà-Tomasi L, Guerrini S, Huang T, Campadelli-Fiume G. 1992. Characterization of human herpesvirus-6(U1102) and (GS) gp112 and identification of the Z29-specified homolog. Virology 191:511–516 [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Okuno T, Isegawa Y, Yamanishi K. 1996. Identification of a variant A-specific neutralizing epitope on glycoprotein B (gB) of human herpesvirus-6 (HHV-6). Virology 222:176–183 [DOI] [PubMed] [Google Scholar]
- 24.Kawabata A, Oyaizu H, Maeki T, Tang H, Yamanishi K, Mori Y. 2011. Analysis of a neutralizing antibody for human herpesvirus 6B reveals a role for glycoprotein Q1 in viral entry. J. Virol. 85:12962–12971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang H, Hayashi M, Maeki T, Yamanishi K, Mori Y. 2011. Human herpesvirus 6 glycoprotein complex formation is required for folding and trafficking of the gH/gL/gQ1/gQ2 complex and its cellular receptor binding. J. Virol. 85:11121–11130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, Arase N, Shiratori I, Tanaka S, Kawaguchi Y, Spear PG, Lanier LL, Arase H. 2008. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell 132:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanarsdall AL, Chase MC, Johnson DC. 2011. Human cytomegalovirus glycoprotein gO complexes with gH/gL, promoting interference with viral entry into human fibroblasts but not entry into epithelial cells. J. Virol. 85:11638–11645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akkapaiboon P, Mori Y, Sadaoka T, Yonemoto S, Yamanishi K. 2004. Intracellular processing of human herpesvirus 6 glycoproteins Q1 and Q2 into tetrameric complexes expressed on the viral envelope. J. Virol. 78:7969–7983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori Y, Seya T, Huang HL, Akkapaiboon P, Dhepakson P, Yamanishi K. 2002. Human herpesvirus 6 variant A but not variant B induces fusion from without in a variety of human cells through a human herpesvirus 6 entry receptor, CD46. J. Virol. 76:6750–6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seya T, Hara T, Matsumoto M, Akedo H. 1990. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J. Immunol. 145:238–245 [PubMed] [Google Scholar]
- 31.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J. Virol. 84:12292–12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. 2011. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 9:369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu DX, Gompels UA, Foa-Tomasi L, Campadelli-Fiume G. 1993. Human herpesvirus-6 glycoprotein H and L homologs are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology 197:12–22 [DOI] [PubMed] [Google Scholar]
- 34.Okuno T, Sao H, Asada H, Shiraki K, Takahashi M, Yamanishi K. 1990. Analysis of a glycoprotein of human herpesvirus 6 (HHV-6) using monoclonal antibodies. Virology 176:625–628 [DOI] [PubMed] [Google Scholar]
- 35.Qian G, Wood C, Chandran B. 1993. Identification and characterization of glycoprotein gH of human herpesvirus-6. Virology 194:380–386 [DOI] [PubMed] [Google Scholar]
- 36.Takeda K, Haque M, Sunagawa T, Okuno T, Isegawa Y, Yamanishi K. 1997. Identification of a variant B-specific neutralizing epitope on glycoprotein H of human herpesvirus-6. J. Gen. Virol. 78(Pt 9):2171–2178 [DOI] [PubMed] [Google Scholar]
- 37.Akula SM, Pramod NP, Wang FZ, Chandran B. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407–419 [DOI] [PubMed] [Google Scholar]
- 38.Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, Imai T, Minowa A, Akashi H, Arase H, Kawaoka Y, Kawaguchi Y. 2010. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature 467:859–862 [DOI] [PubMed] [Google Scholar]
- 39.Feire AL, Roy RM, Manley K, Compton T. 2010. The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry. J. Virol. 84:10026–10037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soroceanu L, Akhavan A, Cobbs CS. 2008. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 455:391–395 [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456–461 [DOI] [PubMed] [Google Scholar]


