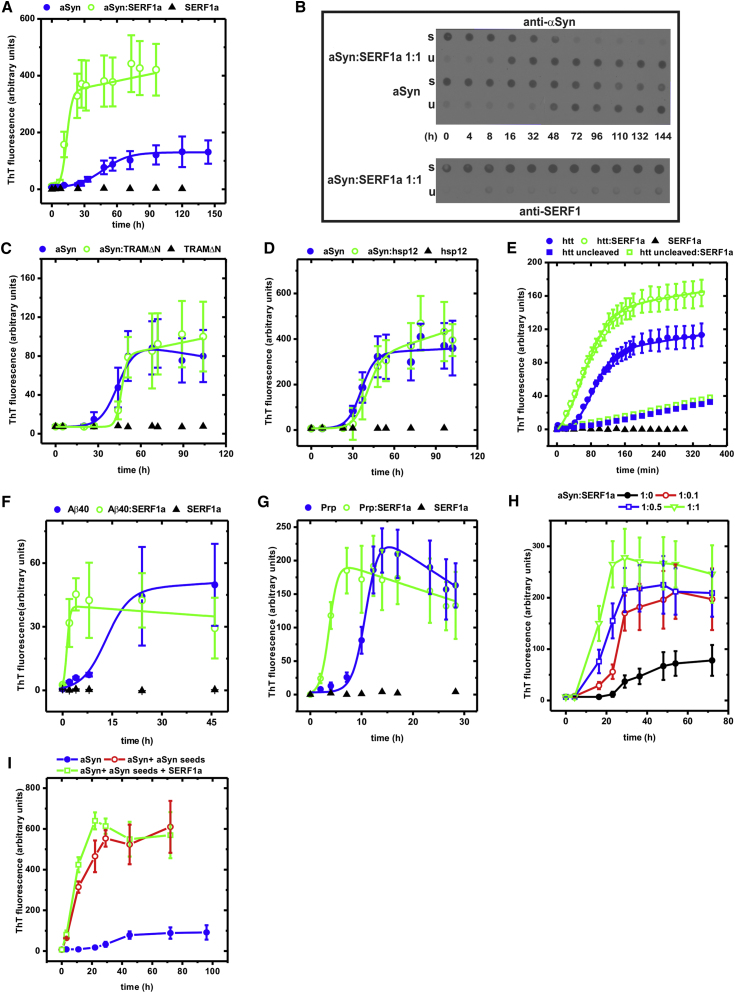
Figure 2.
Amyloid-Promoting Properties of SERF1a
(A) ThT-monitored amyloid kinetics of aSyn in the absence (closed blue circle) and in the presence of SERF1a (open green circle).
(B) Dot-blot partition analysis/immunodetection of aSyn and SERF1a in sample aliquots drawn during the aSyn amyloid growth reaction. Upper (detection of aSyn): In the presence of equimolar amounts of SERF1a, the formation of unsoluble fibers was accelerated and after 48 hr aSyn relocated almost quantitatively from the soluble (s) into the unsoluble (u) fraction. In the absence of SERF1a, the formation of unsoluble aSyn species was slower. An amount of soluble aSyn was still detectable even at t ≥ 48 hr, indicating that the conversion was not quantitative. Lower (same samples; detection of SERF1a): SERF1a remained soluble during aSyn amyloid fiber growth, indicating that itself does not aggregate or coprecipitate with amyloid fibers (see also Figure S1A).
(C and D) Amyloid kinetics of 100 μM aSyn in the absence (closed blue circle) and in the presence (open green circle) of equimolar amyloid-unrelated control proteins TRAMΔN (C) and hsp12 (D) (Ex/em slit widths = 10/20). Amyloidogenesis was insignificantly affected.
(E) Amyloid kinetics of GST-httQ53Ex1 in the absence (closed blue circle) and in the presence of SERF1a (open green circle). Fiber formation was initiated by the proteolytic cleavage of the GST-tag (Wacker et al., 2004). Without cleavage of the GST tag, amyloidogenesis did not occur (closed blue square in the absence of SERF1a; open green square in the presence of SERF1a). The weak signal increase is possibly due to autoproteolysis and the resulting formation of little amounts of amyloids.
(F) Amyloid kinetics of Aβ40 in the absence (closed blue circle) and in the presence of SERF1a (open green square).
(G) Amyloid kinetics of PrP in the absence (closed blue circle) and in the presence of SERF1a (open green square).
(H) Amyloid kinetics of 100 μM aSyn in the absence (closed black circle) and in the presence of different SERF1a ratios demonstrates that substoichiometric amounts of SERF1a are sufficient to enhance amyloidogenesis.
(I) A concentration of 1.5 μg/ml aSyn nucleation seeds, generated by ultrasonication of mature aSyn fibers, promotes the amyloid conversion of 100 μM aSyn (1.5 mg/ml) (open red circle). The addition of 100 μM SERF1a marginally influences seeded amyloidogenesis (open green square). (Closed blue circle) 100 μM aSyn in the absence of seeds.
See also Figure S1. The error bars are mean averages, including SEM of three independent experiments.