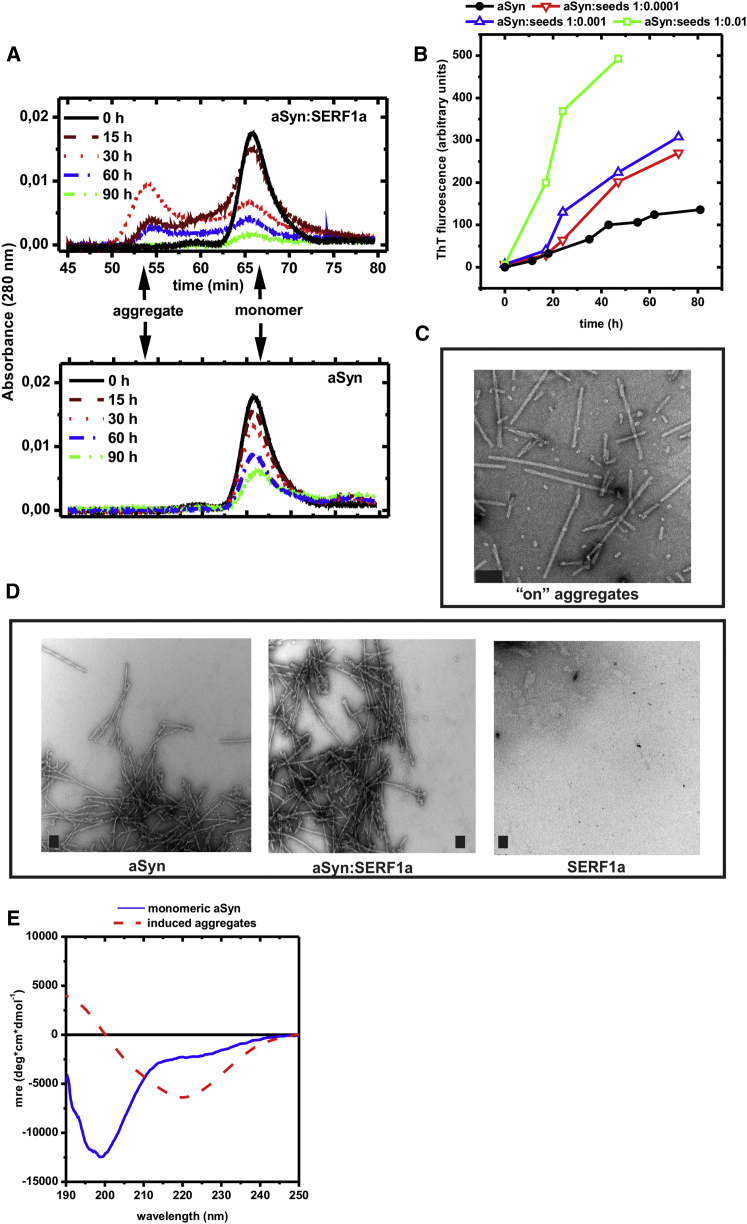
Figure 4.
SERF1a Induces Transiently Stable High Molecular Size aSyn Aggregates
(A) Size-exclusion chromatograms of soluble fractions of 150 μM aSyn, collected at different time points after agitation at 37°C, 1,400 rpm in the presence (upper) and in the absence (lower) of 100 μM SERF1a. Centrifuged samples were run on a Superdex 75 column, equilibrated with 50 mM Tris and 150 mM NaCl (pH 7.4) at 1 ml/min flow rate. SERF1a did not contribute to the absorbance signal, due to the lack of aromatic residues, and it was not present in the aggregate fraction (Figure S3C). Upper: SERF1a-induced aSyn aggregates accumulated over time, in concomitance to a decrease in monomeric aSyn, with a maximum accumulation around 30–40 hr (red dotted curve). After this time point, aggregates decreased in parallel to monomeric aSyn, indicating their consumption into unsoluble fibers (see also Figure 2B). Lower: In the absence of SERF1a, high molecular size aggregates were virtually undetectable, and the consumption of soluble monomeric aSyn was slower and less quantitative.
(B) The isolated aggregates acted as nucleation templates for aSyn fibrils: seeding a 100 μM (1.46 mg/ml) aSyn solution with different ratios of these aggregates caused an acceleration of fiber formation (closed black circle, open green square, open upward blue triangle, and open downward red triangle).
(C) Transmission electron micrograph of the isolated aSyn intermediates display a morphology consisting of small dots of 20–30 nm diameter and short rods of varying length (50–400 nm). Scale bars = 100 nm.
(D) Transmission electron micrographs of mature aSyn amyloid fibers obtained in the absence (left) and in the presence (middle) of SERF1a showed no morphological difference. SERF1a alone did not aggregate (right). Scale bars = 200 nm.
(E) Far-UV CD-spectrum of monomeric aSyn (solid blue line) and of soluble aSyn aggregates (solid red line). Although the spectrum of monomeric aSyn is typically unstructured (lack of predominant alpha helix or beta sheet signal, strong negative signal around 200 nm, and a slow positive signal recovery below 200 nm), the intermediate aggregates are structured, as shown by a prominent signal decrease around 215–220 nm and by a positive signal increase below 200 nm.
Signal deconvolution analysis (Whitmore and Wallace, 2008; Sreerama et al., 1999) yielded 9.9% alpha helix, 34.5% beta-sheet, and 56% random coil for the aSyn aggregates versus 6.4% alpha helix, 9.8% beta sheet, 82.9% random coil for monomeric aSyn, attesting a predominant structural rearrangement into beta-sheets. See also Figure S3.