Figure S1.
SERF1a Does Not Form SDS-Resistant and/or Unsoluble Aggregates, Related to Figure 2
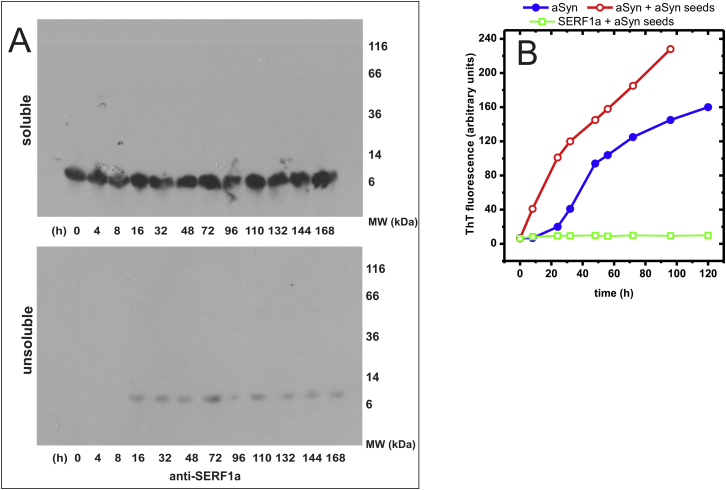
(A) SDS-PAGE resolved partition analysis and immunodetection of SERF1a during the aSyn:SERF1a amyloid growth reaction (same samples as in Figure 2B). The two panels show that SERF1a localizes to the soluble fraction (upper panel), and minimally to the unsoluble fraction (lower panel), indicating that it does not precipitate, co-aggregate with aSyn or form SDS-resistant oligomers during amyloid growth (see also Figure 2B).
(B) 1.5 μg/ml aSyn nucleation seeds, generated by ultrasonication of mature aSyn fibers, promote the amyloid conversion of 100 μM aSyn (1.5 mg/ml) (open red circle), but not of 100 μM SERF1a (open green square). This excludes a hypothetical amyloidogenic cross-seeding of SERF1a, as observable between diverse amyloidogenic proteins (Yagi et al., 2005), in support of intrinsically nonamyloidogenic SERF1a. (Closed blue circle) 100 μM aSyn in the absence of seeds.