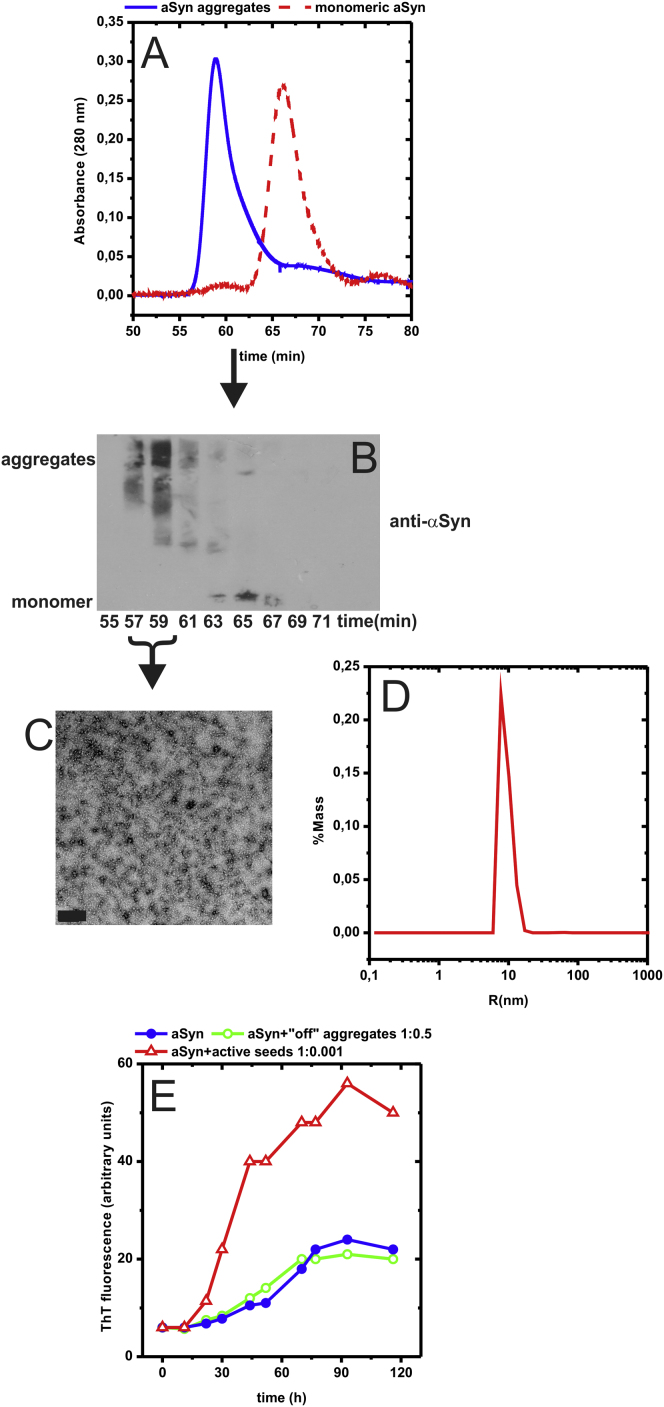
Figure S2.
Purification Scheme of Representative aSyn “Off-Pathway” Aggregates, Related to Figure 3
(A and B) The aggregates were produced by a well described reaction between aSyn and the neurotransmitter dopamine (Cappai et al., 2005; Pham et al., 2009). This small molecule induces the generation of stable, “dead-end” aggregates, which are apart from the amyloid pathway. These were purified by size exclusion chromatography, and eluted faster than monomeric aSyn (A). In agreement to literature (Cappai et al., 2005), the oligomers were SDS-resistant, as shown by SDS-PAGE/immunoblotting of the corresponding chromatographic fractions (B).
(C and D) TEM analysis (C) and dynamic light scattering (D) identified them as spheres with a diameter of approximately 20 nm.
(E) The isolated aggregates did not act as nucleation templates (E), consistent with their “off-pathway” nature. Even at a nearly stoichiometric ratio (100 μM monomeric aSyn:50 μM aggregate) they did not have any effect on aSyn amyloid growth (open green circle). As a control, little amounts of active nucleation seeds generated by ultrasonication of mature aSyn fibers, were sufficient to improve amyloidogenesis (100 μM monomeric aSyn:0.1 μM seed) (open upward red triangle).