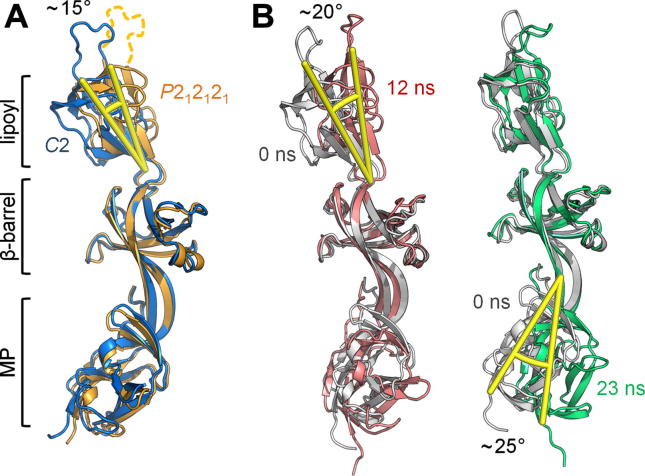
Fig. 3.
Interdomain movement of BesA hinged at the flexible linkers. Structures were superposed over residues 60–71 and 150–229 (i.e. the β-barrel domain) using superpose in the CCP4 suite. (A) Comparison of BesA monomers crystallised in C2 (blue) and P212121 (orange) space groups. Relative interdomain movement is measured in degrees. Unmodelled loop residues are shown as dotted lines. (B) Molecular dynamics (MD) simulation of BesA. The starting model (0 ns, grey from P212121) is superposed to the main chain of snapshots from the MD trajectories at 12 ns (red) and 23 ns (green).