Fig. 4.
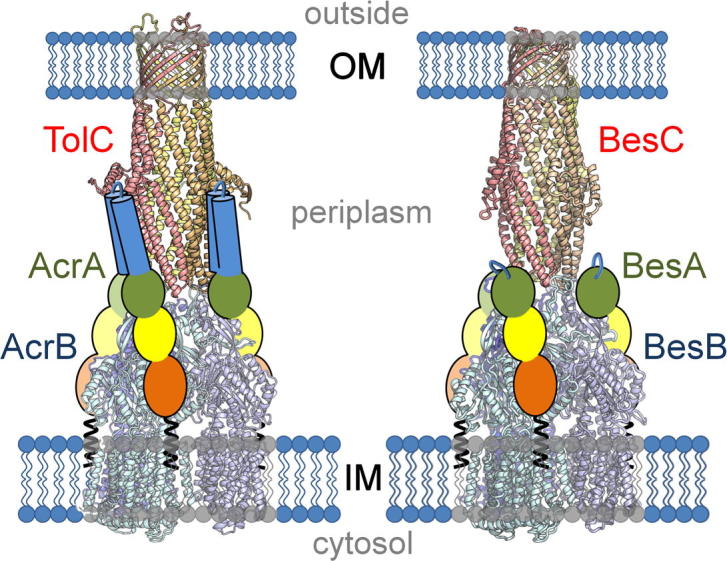
Predicted assembly of the tripartite BesABC pump of B. burgdorferi. Left, assembled E. coli TolC (red)-AcrA (coloured as in Fig. 2)-AcrB (blue) pump, based on in vivo site-specific cross-linking and data-based multidomain docking [7]. Right, predicted assembly of B. burgdorferi BesC (homology model, red)-BesA (coloured as in Fig. 2)-BesB (homology model, blue), based on the E. coli model. The simplest 1:1:1 ratio of BesA-BesB-BesC is shown, based on AcrA-AcrB-TolC [7]. A 2:1:1 ratio has also been suggested in which 6 adaptors form a ring round the transporter mediated by contacts through the β-barrel and lipoyl domains, as seen in the in vitro co-crystallised CusBA subcomplex [3,35,37]. The absence of an adaptor α-hairpin in Borrelia BesA is not compensated by extra domains in the cognate IM (BesB) or OM (BesC) pump components.
