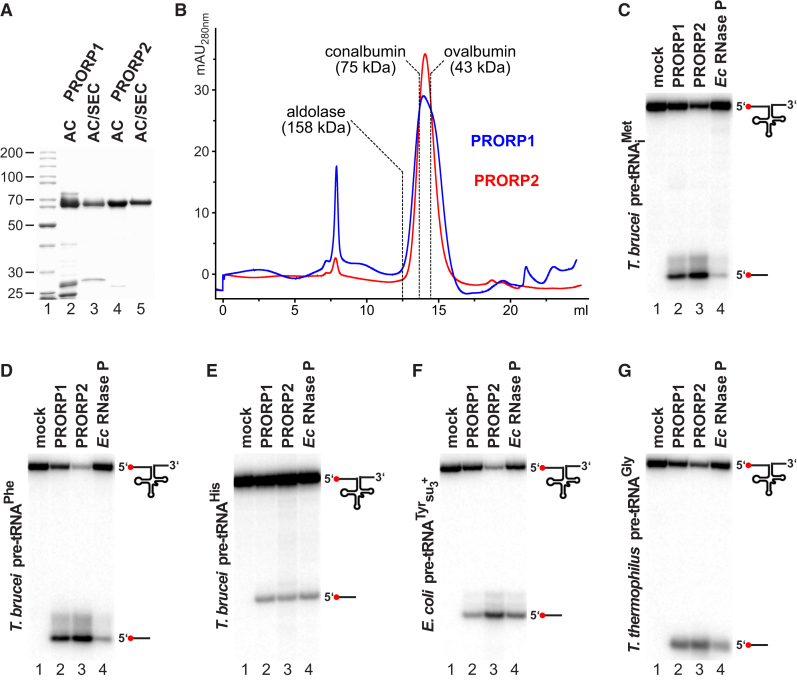
Figure 1.
Purification and tRNA-Processing Activity of Recombinant T. brucei PRORP1 and PRORP2
(A) SDS-PAGE of recombinant PRORP1 and PRORP2 purified by affinity chromatography (AC), or affinity chromatography with subsequent size exclusion chromatography (SEC) is shown; molecular weight of marker proteins indicated in kDa.
(B) Size exclusion chromatography profile of purified recombinant PRORP1 (blue) and PRORP2 (red) is illustrated. The peak at about 8 ml represents aggregated protein eluting in the void volume. The peak positions of molecular weight marker proteins resolved under identical conditions are indicated.
(C) RNase P activity of recombinant PRORP1 and PRORP2 is presented. A T. brucei tRNAiMet precursor was incubated with PRORP1, PRORP2, or E. coli RNase P. 5′ end-labeled substrate RNA and cleavage product (indicated by icons to the right) were resolved by denaturing PAGE.
(D) Same as (C), but T. brucei tRNAPhe precursor used as substrate is shown.
(E) Same as (C), but T. brucei tRNAHis precursor used as substrate is presented.
(F) Same as (C), but E. coli tRNATyrsu3+ precursor used as substrate is illustrated.
(G) Same as (C), but tRNAGly precursor from Thermus thermophilus used as substrate is demonstrated.
See Figure S1 for alignment of trypanosomatid PRORP sequences to human and plant PRORP.