Abstract
Purpose
[18] Fluoro-deoxyglucose-positron-emission tomography/computerized tomography (FDG-PET) is commonly used to assess response to patients treated with radiation (RT) or Chemotherapy and RT (CRT). The intent of this pilot study is to explore whether FCH-PET can serve as an early predictive biomarker for early detection of RT/CRT response.
Materials/Methods
Fourteen patients have been accrued and analyzed. The lesions were base of tongue, tonsil, nodes, hypopharynx, maxilla, palate, lung, pancreas, brain, uterine and rectal. There were 16 lesions that were considered target lesion and were followed for correlation between change in FCH-PET SUVmax readings and clinical outcome. Median tumor size was 4.4 cm. Median RT dose was 66Gy. The change in SUVmax (Δ SUVmax) of FCH-PET scans performed before and during RT was correlated with clinical outcome at the last follow-up.
Results
The median FCH-PET SUVmax for the 1st and 2nd scans was 6.15 and 4.65 respectively. Fourteen (87.5%) lesions showed a reduction in SUVmax in either a CR/PR (complete/partial response) and 2 lesions showed an increase in SUVmax both of which were determined to be NR. The median percentage change between the 1st and 2nd scan was −19.5%. Forty-four percent lesions (7/16) had CR, 44% (7/16) had PR and 12% (2/16) had NR (No response). Median follow-up was 12 months. The results showed a difference between NR and PR, between NR and CR, showed a trend towards significance (p=0.06).
Conclusion
FCH-PET scan demonstrated changes in SUVmax during RT that were predictive of final outcome
Keywords: Choline, PET, Radiation, response, FDG
Introduction
Definitive RT, chemotherapy (CT), or a combination of the two (CRT) with or without surgical resection are being employed in the management of primary or metastatic malignancies of head and neck (HN), central nervous system (CNS), gynecological (GYN), Gastrointestinal (GI), Genitourinary (GU) and chest, as dictated by the respective stage of the cancer. [18]Fluoro-deoxyglucose-positron emission tomography/computerized tomography (FDG-PET) is commonly used to assess response to these treatments (1). Several studies have looked at the role of FDG-PET in assessment of RT response and have found a wide range of predictive values (14–91%) (2). In HN cancers, FDG-PET is able to detect residual nodal disease in patients with a partial response to complete response (CR) with a reported sensitivity of 71% to 100%, specificity of 43% to 100%, positive predictive value of 46% to 100%, and negative predictive value of 66% to 100%.(3–9). A time interval of 8–12 weeks after RT or CRT is required for a restaging by FDG-PET to avoid normal tissue edema or inflammation which has been shown to contribute to false-positive and false-negative results (10, 11). Therefore, there is a need for a test that is able to uncover the ultimate RT/CRT response at the early phase of the treatment course. Such a test may render clinical significance by potentially avoiding subjecting the patient to surgical resection and its related toxicities if no tangible therapeutic benefit is being derived.
One promising non-invasive test is [18F]Fluorocholine PET (FCH-PET). Choline is a precursor of phosphatidylcholine that is a major constituent of membrane lipids. When choline enters tumor cells, it undergoes phosphorylation and after several biosynthetic processes, finally becomes integrated into lecithin, a major component of cell membrane phospholipids. The biosynthesis of cell membranes is rapid in tumor cells because of fast replication of tumor cells and therefore, the choline uptake is higher in these cells. Radiolabeled choline has been shown to be taken up higher in the tumor cells than in fibroblasts and has been shown to correlate with the proliferative activity of tumor cells (12). FCH-PET has demonstrated its favorable imaging characteristics as a diagnostic/staging tool. For example, in musculoskeletal tumors, a comparison of FDG-PET with [11C]-choline-PET showed that [11C]-choline-PET may equivalent to FDG-PET for differentiating malignant from benign tumors, though the advantages of [11C]-choline-PET were shorter examination time and little retention in the bladder (13) that may be particularly beneficial in gynecological cancers (14).
We hypothesized that FCH-PET tumor uptake at 3–4 weeks into RT/CRT will predict final treatment response in patients. We performed a pilot study is to quantify variance in signal changes between FCH-PET images taken before and during RT/CRT and to determine its correspondence with tumor response at a few months after the completion of therapy.
Materials and Methods
The prospective study was approved by the institutional review board (IRB) and patients consent was obtained prior to enrollment in the study. Patients: From April 2009 till June 2010, 14 patients with malignancies of head and neck, lung, esophagus, rectum and brain were included in the study. Stage I-IV was included (AJCC staging, 6th edition). Patients were staged prior to enrollment using a PET-CT, CT or an MRI and a biopsy was performed to confirm malignancy in all patients. Treatment: Patients were treated with RT/CRT based on the stage, prior history and overall performance status (Table 2). Study Design: After the initial staging scans for all patients (standard of care), FCH-PET scan was performed prior to beginning RT/CRT. A second FCH-PET scan was performed within 3–4 weeks of starting treatment. After completing treatment, each patient underwent imaging to assess response (CT and/or MRI and/or PET/CT) or surgical resection (eg. rectal cancer) as deemed to be the standard of care for each type and stage of cancer. The variation in the two FCH-PET readings was correlated with the final clinical outcome. Wilcoxon rank-sum tests were employed to compare SUV changes between different response groups. FCH-PET image acquisition: The imaging studies were performed at CBIC using a PET/CT system (Discovery LS; GE Medical Systems, Milwaukee, WI) with an in plane resolution of 4.8mm full width at half maximum. A total of 300–400 mBq of 18F radiotracer was injected intravenously. A PET imaging study was started at 15 min for FCH and at 1 hour for FDG study. For attenuation correction, non-diagnostic CT was acquired just prior to PET scanning. Following the CT acquisition, a PET scan was acquired in 2-D mode (4–5 min/bed and 2–4 beds as needed). Both attenuation-corrected and non-attenuation-corrected images of FCH and FDG PET data were qualitatively compared for uptake in the malignant and normal tissues by two independent nuclear medicine physicians.
Table 2.
Patient Treatment Characteristics
| Size (cm) | 4.4 (median) |
|---|---|
| Definitive RT | 12 patients |
| Palliative RT | 2 patients |
| RT dose | 66Gy (median) |
| Concurrent chemo | 11 patients |
Results
Patients and tumor characteristics
The patient and tumor characteristics are summarized in Table 1. Fourteen patients have been accrued to date and analyzed. There were 8 males and 6 females. The median age was 65 years (range 45–84 years). There were 16 lesions in 14 patients that were considered target lesion and were followed for correlation between change in FCH-PET SUVmax readings and clinical outcome. Median tumor size was 4.4 cm (range 1.9–13cm). Twelve patients received definitive RT and 2 patients (1 lung, 1 maxilla) received palliative RT. Clinical outcome was measured using clinical exam and/or imaging as well as pathological assessment of resected specimens. Response was defined as either complete response (CR), less than complete response-partial response (PR), and no change or progressive disease was described as no response (NR).
Table 1.
Patient Characteristics
| Patient # |
sex | age | Site | stage | Histology | Follow-up (months) |
|---|---|---|---|---|---|---|
| 1 | m | 57 | BOT | IV | Squamous cell | 16 |
| hypopharynx | IV | Squamous cell | ||||
| rt LN | IV | Squamous cell | ||||
| 2 | m | 84 | palate | IV | Squamous cell | 1 |
| 3 | m | 72 | BOT | III | Squamous cell | 14 |
| 4 | m | 71 | Lung | IV | Adenocarcinoma | 5 |
| 5 | f | 62 | lung | IIIB | Adenocarcinoma | 13 |
| 6 | f | 70 | Lung | IV | Squamous cell | 3 |
| 7 | f | 62 | esophagus | IV | Adenocarcinoma | 13 |
| 8 | m | 65 | maxilla | IV | Squamous cell | 1 |
| 9 | m | 46 | BOT | I | Squamous cell | 13 |
| 10 | m | 78 | pancreas | III | Adenocarcinoma | 4 |
| 11 | m | 71 | rectal | IV | Adenocarcinoma | 12 |
| 12 | f | 65 | brain | IV | Gliosarcoma | 12 |
| 13 | f | 45 | Lung | IIIB | Adenocarcinoma | 9 |
| 14 | f | 67 | uterine | II | Adenocarcinoma | 6 |
Change in SUVmax of the two FCH-PET scans
The median FCH-PET SUVmax for the 1st and 2nd scans was 6.15 and 4.65 respectively (Figure 1). Fourteen (87.5%) lesions showed a reduction in SUVmax in either a CR/PR (PPV 100%) and 2 lesions showed an increase in SUVmax both of which were determined to be NR. The median percentage change between the 1st and 2nd scan was −19.5%. Forty-four percent lesions (7/16) had CR, 44% (7/16) had PR and 12% (2/16) had NR. Median follow-up was 12 months (range 1–16 months). Clinical Response and change in FCH-PET readings are shown in Table 3. Wilcoxon rank-sum tests were employed to compare SUV changes between different response groups. The results showed a difference between NR and PR, and between NR and CR, showed a trend towards significance (p=0.06). However, if the sign of the differences between the two SUVmax values is used as variable, Fisher's exact test shows a statistical significant association with the response (p=0.03, for both comparisons: NR vs PR, CR vs NR) thereby suggesting correlation between change in FCH-PET SUVmax during RT and clinical outcome. While we found these results encouraging, we must stress that only 2 samples were collected that had NR as response. A larger collection of samples is thus necessary to confirm the results of our study.
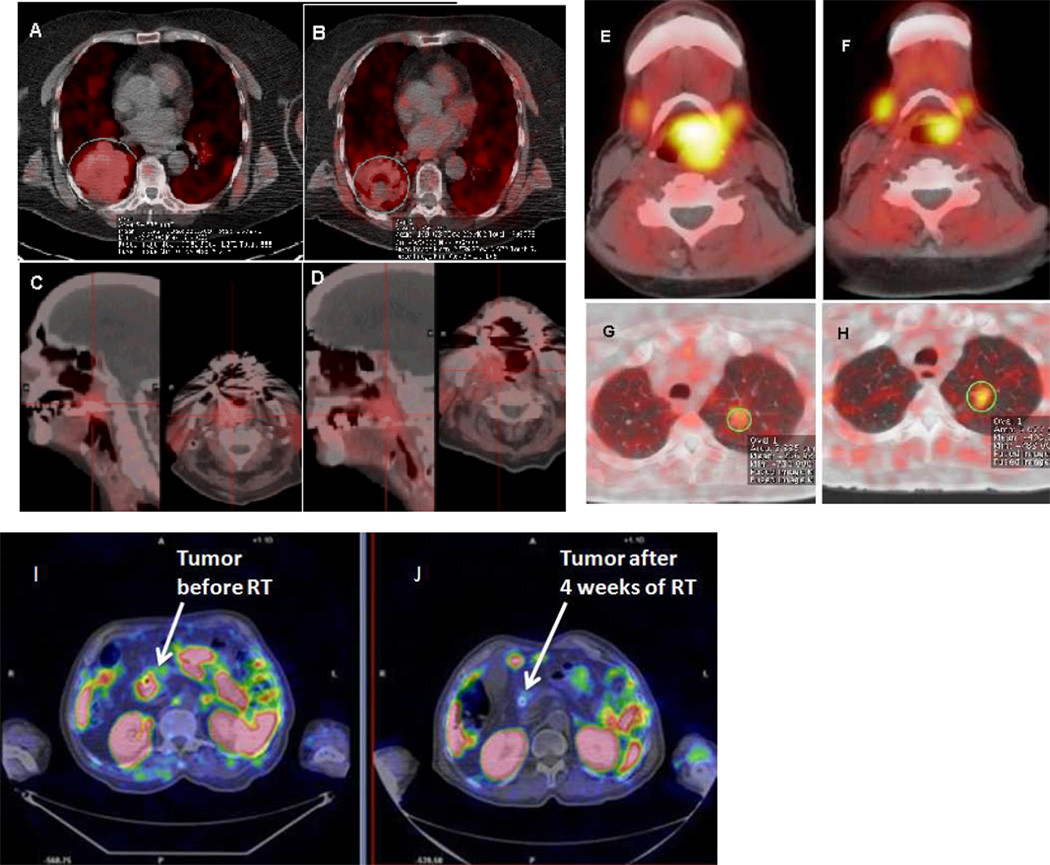
Figure 1.
FCH-PET scans performed prior to RT and then during RT showing treatment response. 1. Sixty-nine year old female with stage IIIB NSCLC, FCH-PET performed prior to RT, SUVmax 6.0 (A) and 3 weeks later, SUVmax of 5.1 (B). 2. Eighty-four year old with stage IV oropharyngeal cancer with FCH-PET SUVmax of 6.5 before RT (C) and 4.9 (D) after 4 weeks of RT. 3. Fifty-seven year old with hypopharynx cancer, with pre-RT FCH-PET SUVmax of 5.3 (E), and 4.4 after 3 weeks of RT (F). 4. Sixty-one year old female with stage III NCLC, showing a pre-RT FCH-PET SUVmax of 2.5 (G), and 4.3 after 4 weeks of RT (H). 5. Seventy-eight year old with un-resectable pancreatic cancer with pre-RT SUVmax of 6.0 (I), and 2.0 after 4 weeks of RT (J).
Table 3.
| Lesion # |
Site | FCH-SCAN 1 READING |
FCH- SCAN 2 READING |
% change | Response at completion of RT |
Post-RT scan |
Clinical outcome after RT |
|---|---|---|---|---|---|---|---|
| 1 | BOT | 6.70 | 5.50 | −18.00 | CR | FDG-PET | Mass not palpable |
| 2 | Hypopharynx | 5.30 | 4.40 | −17.00 | CR | FDG-PET | No mass seen |
| 3 | Rt Neck node | 3.70 | 3.60 | −3.00 | CR | FDG-PET | No mass seen |
| 4 | Palate | 6.50 | 4.90 | −25.00 | CR | CT | Pt died from complications |
| 5 | BOT | 5.10 | 3.80 | −26.00 | CR | FDG-PET | Mass not palpable |
| 6 | Lung | 6.30 | 5.00 | −21.00 | PR | CT | Hemoptysis stopped |
| 7 | Lung | 2.50 | 4.30 | 72.00 | NR | FDG-PET | Stable disease |
| 8 | Lung | 6.00 | 5.10 | −15.00 | PR | FDG-PET | Developed DM |
| 9 | esophagus | 8.50 | 6.30 | −26.00 | PR | CT | Dysphagia significantly improved |
| 10 | maxilla | 9.90 | 5.30 | −47.00 | CR | CT | Developed DM |
| 11 | BOT | 5.20 | 4.40 | −16.00 | CR | FDG-PET | Mass not palpable |
| 12 | Pancreas | 6.00 | 2.00 | −67.00 | PR | CT, CA-19-9 | Developed DM, persistent disease |
| 13 | Rectal | 17.60 | 1.50 | −93.00 | PR | surgery | Residual disease in surgical specimen |
| 14 | Brain | 0.20 | 0.50 | 150.00 | NR/PD | MRI | Developed local recurrence |
| 15 | Lung | 9.40 | 8.20 | −13.00 | PR | FDG-PET | Developed DM |
| 16 | Uterus | 9.30 | 5.60 | −40.00 | PR | FDG-PET | Local recurrence |
In addition, there was minimal non-specific uptake noted on the 2nd FCH-PET scan thereby minimizing false positive rates as seen on FDG-PET.
In addition to the FCH-PET scans performed at 3–4 weeks after starting RT, clinical exams were also performed as surrogate evaluation to check tumor response. For each patient enrolled in the study, the clinical outcome at the last follow-up (f/u) was as follows: Patient 1: Locally advanced HN cancer, FCH-PET showed a 18%, 17% and 3% reduction in SUVmax for each site respectively at 3 weeks of RT. Clinical exam performed at the time of the 2nd FCH-PET scan showed a reduction in the size of tumors at each primary location and the nodes. Pre-RT FDG-PET showed an SUVmax of 18.7, 20.9 and 15.4 respectively. These treated areas were non-hypermetabolic after completion of CRT. The patient is disease free in the head and neck area at the time of last f/u but has developed a new primary metastatic lung cancer (adenocarcinoma) that has been treated with chemotherapy. Patient 2: Locally advanced soft palate cancer, 3.8cm tumor with a pre-RT SUVmax was 24.5. FCH-PET showed a 25% reduction at 4 weeks of RT. Clinical exam showed a reduction in the size of tumor at the same time. A clinical exam confirmed complete resolution of all tumors at the primary site and nodes. However, patient died 1 month after treatment due to complications arising from his treatment and multiple co-morbidities. Patient 3: Patient with stage IV non-small cell lung cancer (NSCLC) presenting with hemoptysis. Patient received a course of RT that resulted in complete resolution of hemoptysis. FCH-PET SUVmax showed a 21% reduction and a decrease in the size of the left bronchial mass. Patient developed brain metastasis that was treated with stereotactic radiation. Patient 4: Locally advanced BOT cancer with a pre-CRT FDG-PET SUVmax of 8.9. FCH-PET showed a 26% reduction in SUVmax at 4 weeks of CRT. Clinical exam at 4 weeks revealed a reduction in size of BOT. No metabolic activity on post-treatment FDG-PET. Patient disease free at last follow-up (f/u). Patient 5: Patient with stage IIIB NSCLC, received CRT, showed 72% increase in SUV of FCH-PET at 4 weeks of RT, showed a persistent mass unchanged in size (5.5cm) and FDG-PET showing an SUVmax of 5.5, 3 months after RT, compared to FDG-PET SUVmax of 3.4 and size 2.1cm prior to RT. Patient was clinically diagnosed with persistent disease. Patient has developed renal cell cancer at the last f/u. Patient 6: Patient with stage IIIB NSCLC, pre-CRT FDG-PET SUVmax of 19.8. Patient received CRT and FCH-PET revealed a 15% reduction in SUVmax at 3 weeks of RT. Post-CRT FDG-PET showed a SUVmax of 6.0. However, patient developed metastatic disease 2 months after completion of treatment. Patient expired 4 months later from brain metastasis. Patient 7: Patient with locally advanced and metastatic esophagus cancer, with a pre-RT FDG-PET SUVmax of 12.6. Patient received CRT, FCH-PET showed a 26% reduction at 4 weeks. There was significant improvement in dysphagia. The patient was followed by serial CT scans, not the FDG-PET scans at the discretion of the treating physicians and showed a complete resolution of the esophageal lesion. However, there was progressive systemic disease and patient is currently on chemotherapy. Patient 8: Patient with locally advanced and twice recurrent maxillary cancer, treated with CRT to a clinically visible symptomatic lesion in the right inner orbit. At 3 weeks, there was a reduction in FCH-PET SUVmax of 47%. There was significant reduction in the size of the visible lesion at the same time. Patient had a clinical complete response at the completion of RT. Patient sent to hospice shortly after due to progressive systemic disease. Patient 9: Patient with history of anaplastic lymphoma, developed a stage I BOT lesion with a pre-RT FDG-PET SUVmax of 14.1, received RT, showed a 16% reduction in FCH-PET SUVmax at 4 weeks with a clinical reduction in tumor size on palpation. Post RT FDG-PET SUVmax was 3.4. The lesion was not palpable post-RT. Patient disease free (BOT) at last follow-up. Patient 10: Patient with un-resectable pancreatic cancer, treated with CRT, FCH-PET SUVmax showed a 67 % reduction at 4 weeks of CRT. Serum CA-19–9 was 135U/ml pre-CRT, 60U/ml during CRT and 68U/ml at 3 months after CRT. Patient had a CT response with a reduction in tumor size from 2.5 to 1.4 cm. However, patient was still considered un-resectable and decided to discontinue all therapy at last f/u. Patient 11: Patient with locally advanced rectal cancer, pre-CRT tumor 8 cm in size and FDG-PET with an SUVmax of 10.4. Patient received CRT followed by surgical resection. Surgical pathology showed a 4cm tumor with negative nodes. Patient developed a single lung metastasis that was resected. Patient was disease free at the last follow-up. Patient 12: Patient received craniotomy for gliosarcoma (WHO grade IV), received RT and Temdar concurrently after resection. FCH-PET performed 4 weeks into CRT showed a small area within the resection cavity that had 150% increase in SUVmax. MRI performed 1 month after RT showed an increase in enhancement in the same area and the patient ultimately developed recurrent disease in the area. The recurrence was surgically removed. Surgical pathology revealed recurrent gliosarcoma. Patient disease free at the last follow-up. Patient 13: Patient with stage IIIB NSCLC, FDG-PET pre-CRT showed a 5.6 cm right lower lobe mass with an SUVmax of 37. Patient received CRT and showed a 13% reduction in FCH-PET SUVmax at 3 weeks. FDG-PET performed a month after completion of CRT showed the right lung mass of 4.6 cm and SUVmax of 19. Patient underwent salvage pneumonectomy that showed a 3.5cm tumor and one positive lymph node. Patient is disease free locally at the last follow-up but developed brain metastasis for which the patient received whole brain RT. Patient 14: Patient had refused surgical resection of her uterine adenocarcinoma (presented with vaginal bleeding), FDG-PET showed a 13 cm tumor with an SUVmax of 10.4 pre-RT. Patient treated with RT only and FCH-PET SUVmax showed a 40% reduction at 4 weeks. Patient reported complete resolution of vaginal bleeding and reduction in size of the uterine mass at the completion of RT. However, patient refused any surgical intervention and ultimately had a progressive disease at 3 months after RT-increase in tumor size to 15cm and increase in FDG-PET SUVmax to 13.8. Patient decided to get further treatment at another institution.
Discussion
Our study showed an excellent correlation between FCH-PET scan performed during RT with the final clinical outcome assessed clinically or with imaging.
PET utilizes positron-emitting annihilation event that occurs when electrons and positrons collide and vanish with two opposed photons with a precise energy of 511KeV. This sort of annihilation reaction can be demonstrated in natural radio-isotopes such as oxygen-15, fluorine-18, and carbon-11. The radionuclide that is most frequently used in clinical practice is fluorine-18 (18F) that has a half life of 110 minutes. The sensitivity of PET scan relates to the fact that glucose analog, fluoro-deoxyglucose (FDG) gets trapped in the cell by phosphorylation. Cancer cells demonstrate significantly increased glucose uptake because of increase expression of glucose transporter enzyme GLUT-1, increased hexokinase activity as well as increased glycolysis. The kinetics of labeled glucose (18FDG) produces increased signal to noise ratio and the intensity of the signal can be measured using the ‘standard uptake value’ (SUV) that normalizes signal size to infused isotope and patient body mass. This rationale is valid for most regions of the body, with the exception of tissues in which glucose metabolism is normally high; these include the brain, heart muscle, skeletal muscle, brown fat and inflammation. FDG tumor imaging is also poor in the region of the bladder, since FDG is excreted into the urine. FDG uptake is also reduced when the blood sugar level is high, as in poorly controlled diabetes.
FDG-PET had certain limitations in assessing RT response early during RT course. In a study performed in head and neck cancer patients to study the accuracy of PET/CT in assessing response to RT, the accuracy of FDG-PET/CT was 85.7%, compared with 67.9% for CT alone. All false-negative and false-positive FDG-PET/CT results occurred between 4 and 8 weeks after treatment. The authors concluded that the metabolic-anatomic information from co-registered FDG-PET/CT provided the most accurate assessment for treatment response when performed later than 8 weeks after the conclusion of radiation therapy (15). A study performed to prospectively assess the value of sequential FDG-PET scans in predicting the response of locally advanced rectal cancer to neo-adjuvant CRT, a 79.2% specificity, 81.2% sensitivity, 77% positive predictive value, 89% negative predictive value and 80% overall accuracy was obtained. In this study, all patients underwent FDG-PET/CT both before CRT and 5–6 weeks after completing CRT (16). A study in tumor bearing mice to evaluate the potential of D-18F-FMT, 18F-FDG, L-11C-methionine (L-11C-MET), and 3'-deoxy-3'-18F-fluorothymidine (18F-FLT), as PET ligands for monitoring early responses to radiotherapy showed that tumor uptake of D-18F-FMT was decreased on day 1 after irradiation at 6, 20, or 60 Gy, and the decrease persisted until day 7. Tumor uptake of 18F-FDG was elevated on days 1 and 3 after irradiation at 2, 6, or 20 Gy, followed by a decrease in uptake on day 7 in mice irradiated at 20 or 60 Gy. Decreased tumor uptake of L-11C-MET was observed only on day 3 after the irradiation. Only for D-18F-FMT were significant positive correlations found between ligand uptakes at all the time points examined and tumor volume on day 14 after various doses of irradiation (17).
Choline based PET scans is being increasingly utilized for management of cancers (18, 19). Choline PET has also been recently used to assess response to hormone therapy in prostate cancers (20). A study published from Germany showed that [(11)C]choline has the potential for use in the early monitoring of the therapeutic effect of docetaxel in a prostate cancer xenograft animal model. The study also indicated that PET with radioactively labeled choline derivatives might be a useful tool for monitoring responses to taxane-based chemotherapy in patients with advanced prostate cancer (21). Choline PET has also been used to assess response to photodynamic therapy in prostate cancer models (22). Biochemically, [11C]Choline is indistinguishable from the naturally occurring choline. However, the half-life of [11C]Choline is only 20 minutes resulting in the limited use in centers equipped with on-site cyclotron. The need for longer lasting tracers led to the development of 18F Choline (FCH) based molecules with a half-life of 110 minutes.
Our study was a pilot study to determine the variance in SUVmax of FCH-PET and assessing its correlation with RT/CRT response. However, there was no surrogate FDG-PET scan done at the time of second FCH-PET scan and no FCH-PET scan was performed after completion of RT/CRT (limited funding). However, the patients were evaluated clinically or with serum markers during RT or CRT. Additional scans would certainly have made the study stronger. We are planning additional studies during and after RT/CRT to further evaluate accuracy and specificity of FCH-PET to determine the early RT/CRT response.
Footnotes
No Conflicts of interest
References
- 1.Kutler DI, Wong RJ, Schoder H, Kraus DH. The current status of positron-emission tomography scanning in the evaluation and follow-up of patients with head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2006;14:73–81. doi: 10.1097/01.moo.0000193182.92568.8d. [DOI] [PubMed] [Google Scholar]
- 2.Moeller BJ, Rana V, Cannon BA, Williams MD, Sturgis EM, Ginsberg LE, Macapinlac HA, Lee JJ, Ang KK, Chao KS, Chronowski GM, Frank SJ, Morrison WH, Rosenthal DI, Weber RS, Garden AS, Lippman SM, Schwartz DL. Prospective Risk-Adjusted [18F]Fluorodeoxyglucose Positron Emission Tomography and Computed Tomography Assessment of Radiation Response in Head and Neck Cancer. J Clin Oncol. 2009 May 30; doi: 10.1200/JCO.2008.19.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan WR, Fee WE, Jr, Le QT, Pinto HA. Positron-emission tomography for surveillance of head and neck cancer. Laryngoscope. 2005;115:645–650. doi: 10.1097/01.mlg.0000161345.23128.d4. [DOI] [PubMed] [Google Scholar]
- 4.Fischbein NJ, AAssar OS, Caputo GR, Kaplan MJ, Singer MI, Price DC, Dillon WP, Hawkins RA. Clinical utility of positron emission tomography with 18F-fluorodeoxyglucose in detecting residual/recurrent squamous cell carcinoma of the head and neck. Am J Neuroradiol. 1998;19:1189–1196. [PMC free article] [PubMed] [Google Scholar]
- 5.Conessa C, Foehrenbach H, Poncet JL. FDG-PET scan in local follow up of irradiated head and neck squamous cell carcinomas. Ann Otol Rhinol Laryngol. 2004;113:628–635. doi: 10.1177/000348940411300806. [DOI] [PubMed] [Google Scholar]
- 6.Farber LA, Benard F, Machtay M, Smith RJ, Weber RS, Weinstein GS, Chalian AA, Alavi A, Rosenthal DI. Detection of recurrent head and neck squamous cell carcinomas after radiation therapy with 2-18F-fluoro-deoxy-D-glucose positron emission tomography. Laryngoscope. 1999;109:970–975. doi: 10.1097/00005537-199906000-00024. [DOI] [PubMed] [Google Scholar]
- 7.McCollum AD, Burrell SC, Haddad RI, Norris CM, Tishler RB, Case MA, Posner MR, Van den Abbeele AD. Positron emission tomography with 18F-fluorodeoxyglucose to predict pathologic response after induction chemotherapy and definitive chemoradiotherapy in head and neck cancer. Head Neck. 2004;26:890–896. doi: 10.1002/hed.20080. [DOI] [PubMed] [Google Scholar]
- 8.Goerres GW, Schmid DT, Bandhauer F, Huguenin PU, von Schulthess GK, Schmid S, Stoeckli SJ. Positron emission tomography in the early follow-up of advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:105–109. doi: 10.1001/archotol.130.1.105. [DOI] [PubMed] [Google Scholar]
- 9.Hanasono MM, Kunda LD, Segall GM, Ku GH, Terris DJ. Uses and limitations of FDG positron emission tomography in patients with head and neck cancer. Laryngoscope. 1999;109:880–885. doi: 10.1097/00005537-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Lonneux M, Lawson G, Ide C, Bausart R, Remacle M, Pauwels S. Positron emission tomography with fluorodeoxyglucose for suspected head and neck tumor recurrence in the symptomatic patient. Laryngoscope. 2000;110:1493–1497. doi: 10.1097/00005537-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Greven KM, Williams DW, 3rd, McGuirt WF, Sr, Harkness BA, D'Agostino RB, Jr, Keyes JW, Jr, Watson NE., Jr Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck. 2001;23:942–946. doi: 10.1002/hed.1136. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto M, Waki A, Obata A, Furukawa T, Yonekura Y, Fujibayashi Y. Radiolabeled choline as a proliferation marker: comparison with radiolabeled acetate. Nucl Med Biol. 2004;31(7):859–865. doi: 10.1016/j.nucmedbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Yanagawa T, Watanabe H, Inoue T, Ahmed AR, Tomiyoshi K, Shinozaki T, Oriuchi N, Endo K, Takagishi K. Carbon-11 choline positron emission tomography in musculoskeletal tumors: comparison with fluorine-18 fluorodeoxyglucose positron emission tomography. J Comput Assist Tomogr. 2003;27:175–182. doi: 10.1097/00004728-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Torizuka T, Kanno T, Futatsubashi M, Okada H, Yoshikawa E, Nakamura F, Takekuma M, Maeda M, Ouchi Y. Imaging of gynecologic tumors: comparison of (11)C-choline PET with (18)F-FDG PET. J Nucl Med. 2003;44:1051–1056. [PubMed] [Google Scholar]
- 15.Andrade RS, Heron DE, Degirmenci B, Filho PA, Branstetter BF, Seethala RR, Ferris RL, Avril N. Posttreatment assessment of response using FDG-PET/CT for patients treated with definitive radiation therapy for head and neck cancers. Int J Radiat Oncol Biol Phys. 2006;65:1315–1322. doi: 10.1016/j.ijrobp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, Gava M, Fanti S, Mariani G, Muzzio PC, Rubello D. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007;34:1583–1593. doi: 10.1007/s00259-007-0426-1. [DOI] [PubMed] [Google Scholar]
- 17.Murayama C, Harada N, Kakiuchi T, Fukumoto D, Kamijo A, Kawaguchi AT, Tsukada H. Evaluation of D-18F-FMT, 18F-FDG, L-11C-MET, and 18F-FLT for monitoring the response of tumors to radiotherapy in mice. J Nucl Med. 2009;50:290–295. doi: 10.2967/jnumed.108.057091. [DOI] [PubMed] [Google Scholar]
- 18.Picchio M, Briganti A, Fanti S, Heidenreich A, Krause BJ, Messa C, Montorsi F, Reske SN, Thalmann GN. The Role of Choline Positron Emission tomography/Computed Tomography in the Management of Patients with Prostate-Specific Antigen Progression After Radical Treatment of Prostate Cancer. Eur Urol. 2010 Sep 15; doi: 10.1016/j.eururo.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Roselli F, Pisciotta NM, Aniello MS, Niccoli-Asabella A, Defazio G, Livrea P, Rubini G. Brain F-18 Fluorocholine PET/CT for the assessment of optic pathway glioma in neurofibromatosis-1. Clin Nucl Med. 2010;35:838–839. doi: 10.1097/RLU.0b013e3181ef0b45. [DOI] [PubMed] [Google Scholar]
- 20.De Waele A, Van Binnebeek S, Mottaghy FM. Response assessment of hormonal therapy in prostate cancer by [11C] Choline PET/CT. Clin Nucl Med. 2010;35:701–703. doi: 10.1097/RLU.0b013e3181e9faf5. [DOI] [PubMed] [Google Scholar]
- 21.Krause BJ, Souvatzoglou M, Herrmann K, Weber AW, Schuster T, Buck AK, Nawroth R, Weirich G, Treiber U, Wester HJ, Ziegler SI, Senekowitsch-Schmidtke R, Schwaiger M. [11C]Choline as pharmacodynamic marker for therapy response assessment in a prostate cancer xenograft model. Eur J Nucl Med Mol Imaging. 2010;37:1861–1868. doi: 10.1007/s00259-010-1493-2. [DOI] [PubMed] [Google Scholar]
- 22.Fei B, Wang H, Wu C, Chiu SM. Choline PET for monitoring early tumor response to photodynamic therapy. J Nucl Med. 2010;51:130–138. doi: 10.2967/jnumed.109.067579. [DOI] [PMC free article] [PubMed] [Google Scholar]