Fig 2.
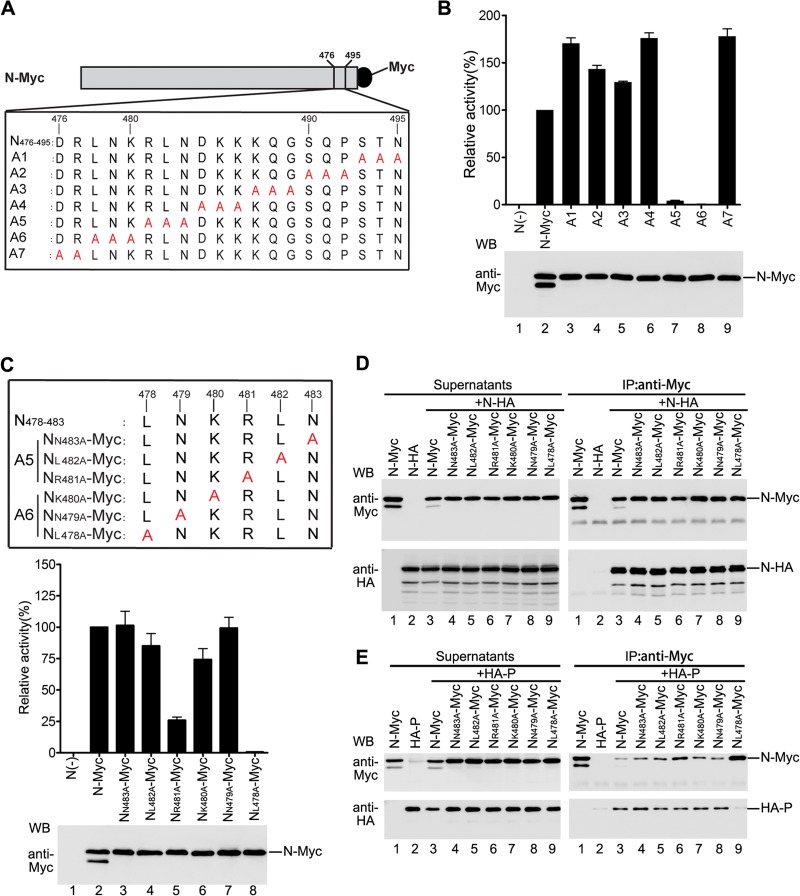
Amino acid L478 within N is critical for the function of N and N-P interaction. (A) Myc-tagged alanine-scanning mutants of N used in this study. Triple amino acids or double amino acids within amino acids 476 to 495 of N were replaced with alanine (shown in red). The names of the mutants are shown on the left. (B) Minigenome RNA synthesis activities of the mutants. Plasmids encoding P, L, minigenome, and N-Myc or mutants were transfected into vTF7-3-infected HeLa cells. Relative luciferase expression levels were measured, and the expression of N-Myc and the mutants was detected via Western blotting as described for Fig. 1F. (C) Minigenome RNA synthesis activities of the alanine-scanning mutants with a single-site mutation within A5 and A6. Amino acids in the region comprising positions 478 to 483 (LNKRLN) of N were replaced with alanine (shown in red). The names of the mutants are shown on the left. Their minigenome RNA synthesis activities were measured as described for Fig. 1F. (D) Interaction of mutants used in panel C with N-HA. Plasmid encoding N-HA was transfected singly or cotransfected with plasmids encoding N-Myc or mutants. Immunoprecipitation was performed with anti-Myc polyclonal antibody. The expression of the proteins was detected by Western blotting using anti-Myc and anti-HA monoclonal antibodies (left). The immune complexes were detected with anti-Myc and anti-HA monoclonal antibodies (right). (E) Interactions between mutants used in panel C and P. Cells were transfected and processed as in panel D, except that the N-HA was replaced by HA-P.