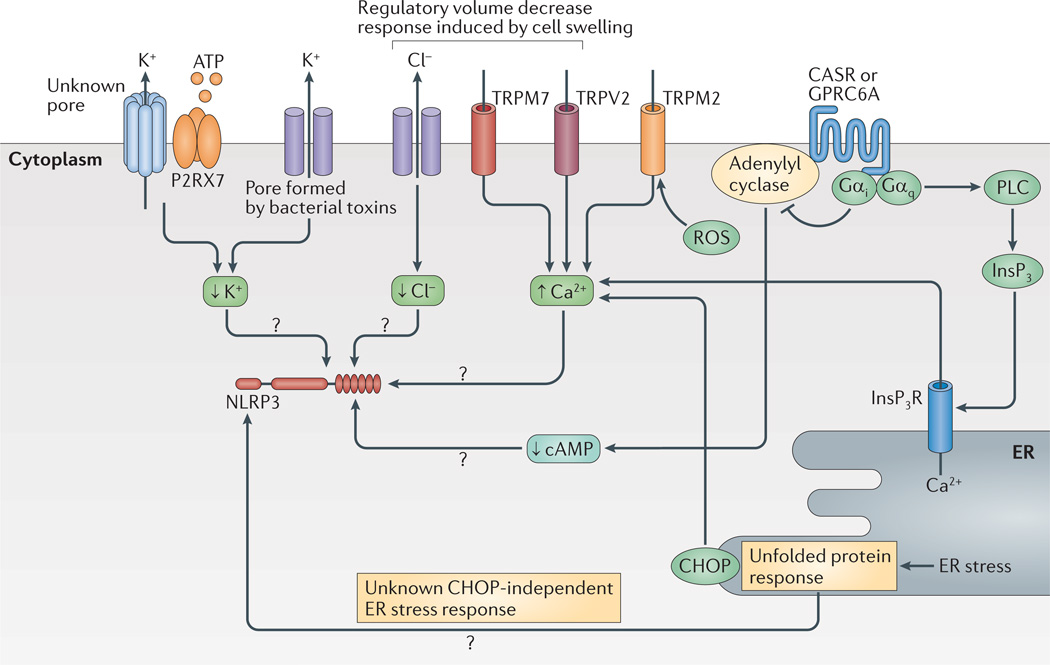
Figure 3. Ion fluxes and cell stress regulate the NLRP3 inflammasome.
K+ and Cl− efflux, as well as Ca2+ mobilization, have a role in the regulation of NLRP3 (NOD-, LRR- and pyrin domain-containing 3). K+ efflux is either achieved directly by pore-forming toxins such as nigericin or indirectly — for example, via the purinergic receptor for ATP P2RX7. Cl− and Ca2+ ion fluxes can be regulated during the regulatory volume decrease response by transient receptor potential receptors (TRPV2 or TRPM7). In addition, reactive oxygen species (ROS) can activate TRPM2 for Ca2+ influx. Ca2+ is also regulated by the unfolded protein response via C/EPB-homologous protein (CHOP). The unfolded protein response is also implicated in other pathways that regulate NLRP3. G-protein coupled receptors (GPCRs) such as calcium-sensing receptor (CASR) and GPRC6A regulate both Ca2+ levels and cyclic AMP (cAMP) levels via activation of phospholipase C (PLC) or inhibition of adenylyl cyclase, respectively. High cAMP levels might directly inhibit NLRP3 (not shown). Inositol-1,4,5-trisphosphate (InsP3) that is generated by PLC leads to release of Ca2+ from the endoplasmic reticulum (ER). How Ca2+ influences NLRP3 activation is not fully understood but it has many molecular targets. Question marks show pathways that are still speculative. InsP3R, InsP3 receptor.