Abstract
Malignant peripheral nerve sheath tumor (MPNST) is a rare soft tissue sarcoma. Most arise in association with major nerve trunks. Their most common anatomical sites are the proximal portions of the upper and lower extremities and the trunk. MPNSTs have rarely been reported in literature to occur in other unusual body parts. We review all such cases reported till now in terms of site of origin, surgical treatment, adjuvant therapy and outcome and shortly describe our experience with two of these cases. Both of our case presented with lump at unusual sites resembling neurofibroma, one at orbitotemporal area and other in the paraspinal region with characteristic feature of neurofibroma with the exception that both had very short history of progression. They underwent gross total removal of the tumor with adjuvant radiotherapy postoperatively. At 6-month follow-up both are doing well with no evidence of recurrence.
Keywords: Malignant peripheral nerve sheath tumor, orbito-temporal, paraspinal, unusual body parts
Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a rare soft tissue sarcoma of the ectomesenchymal origin. It is the malignant counterpart of benign soft tissue tumors like neurofibromas and schwannomas and may follow them. It usually arises from peripheral nerves or somatic soft tissues.[1] Common sites include deeper soft tissues, usually in the proximity of a nerve trunk. MPNSTs can develop in any anatomical region, but the sciatic nerve is affected most often.[2] MPNSTs involving other body parts are extremely rare. Few such lesions have been reported till date. The incidence of MPNST in the general population is 0.001%; however, it can increase to 5-42% in patients with neurofibromatosis type 1 (NF 1). MPNST arising de-novo at an unusual site without any features of NF 1 as has been noticed in our cases is interesting to report.
Case Reports
Case 1
A 35-year-old woman presented with 2-month history of rapidly progressive painless swelling in left orbitotemporal region with proptosis and blurring of vision leading to complete blindness. Physical examination revealed a lobular nontender, firm mass of size 15 × 7 cm extending from left orbit to the left temporal region. In addition to axial proptosis, the left eye showed restricted movement in all directions [Figure 1a and b]. She was unable to perceive light in her left eye. Magnetic resonance imaging (MRI) of the orbits and brain showed left sphenoidal-based extra-axial marginated in homoginously enhancing mass at the lateral side of the left optic nerve buckling the ipsilateral anterotemporal lobe [Figure 2]. Other systemic observations of the patient were normal. Fine needle aspiration cytology came to be neurofibroma. Near total dissection of the tumor was done through a left lateral orbitozygomatic approach. The histopathology and immunohistochemistry of the lesion revealed MPNST [Figure 3a and b]. Postoperatively, the patient recovered rapidly with improved cosmoses [Figure 1c]. Vision in her left eye improved to finger counting at 1 m and extraocular movements were normal. She received local radiotherapy. On 6-month follow-up, the patient is doing well with no local recurrence or any distant metastasis.
Figure 1.
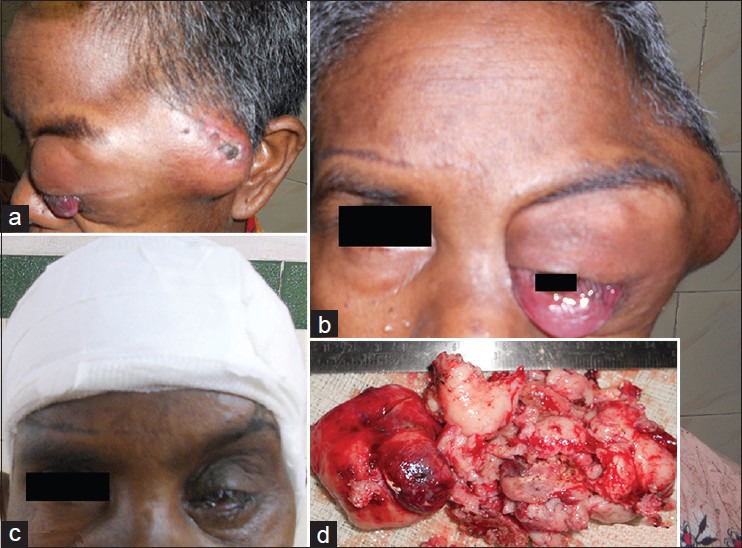
(a) Preoperative photograph. (b) showing the orbitotemporal lump with proptosis. (c) Postoperative cosmesis and (d) Excised tumor mass
Figure 2.
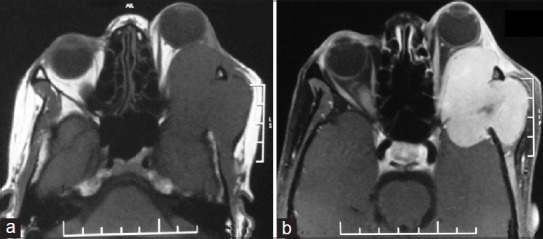
Magnetic resonance imaging of the orbit and brain showing left sphenoidal-based extra-axial plane (a) and contrast (b) marginated in-homoginously enhancing mass at the lateral side of the left optic nerve buckling the ipsilateral antero temporal lobe
Figure 3.
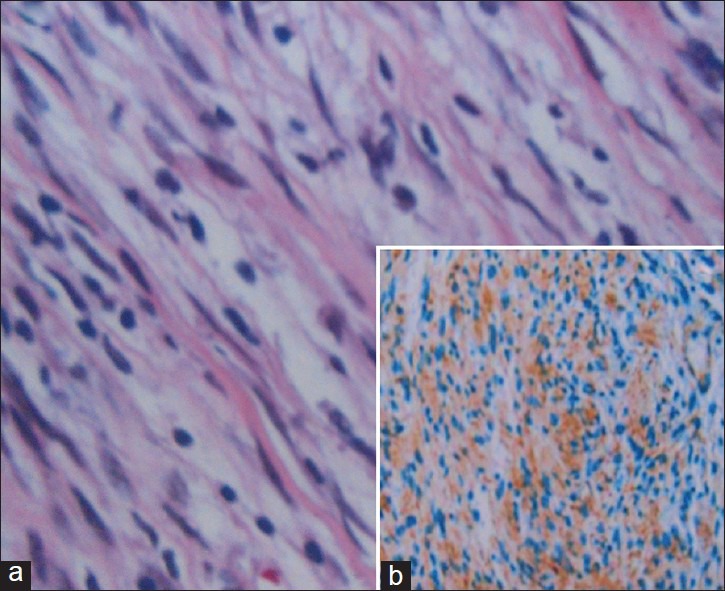
(a) Histopathology picture showing fascicles of spindle cells with marked hypercellularity and high mitotic activity suggestive of MPNS. (b) Immunohistochemistry demonstrates S-100 protein staining of the tumor tissue
Case 2
A 60-year-old male presented with rapidly enlarging painless swelling in back with lower limb weakness in a period of 2 month. On examination, lower motor type of paralysis was found in both the legs with power: 0/5 around all joints. Sensation of all modalities decreased below L3. A nontender hard lobulated mass of size 10 × 5 cm was found over left lumbar paraspinal area fixed to underlying structure [Figure 4a]. MRI was suggestive of lumbar (L1-L4) extradural lesion with associated L3 vertebral body compressional collapse giving a picture of neurofibroma [Figure 5]. Near total excision of both intraspinal and paraspinal component was achieved. Histopathological examination and immunohistochemical staining confirmed the diagnosis of MPNST. Patient improved neurologicaly with power 2/5 around all joints in lower limb. On completion of local radiotherapy at 6-month follow-up, the patient was doing well with no local or systemic spread.
Figure 4.
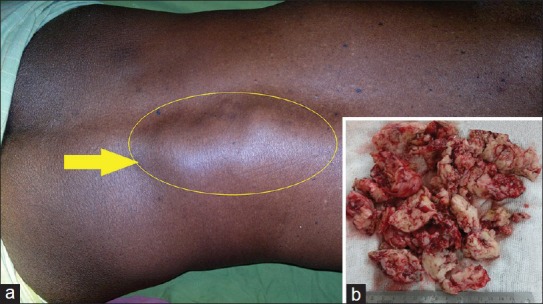
Clinical photograph (a) of case-2 showing the left lumbar mass (Yellow arrow) with excised tumor tissue (b)
Figure 5.
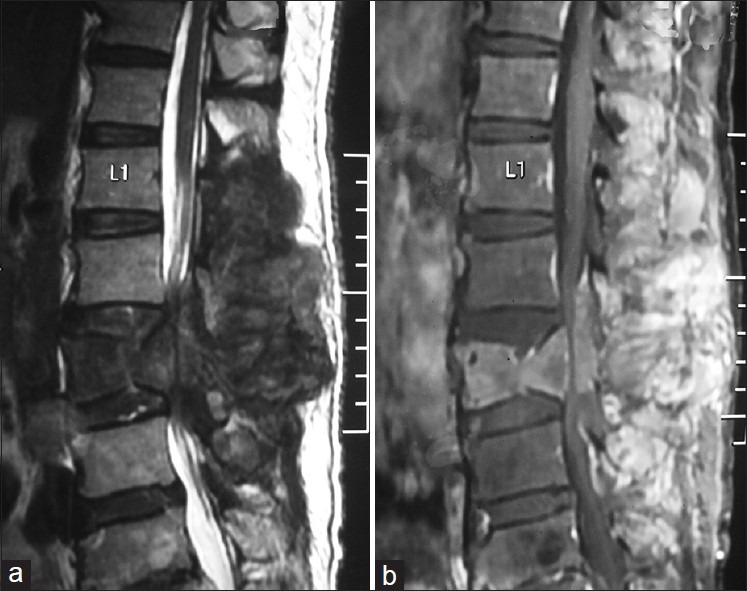
Magnetic resonance imaging of spine in sagittal view shows a T1 (b) hypo, T2 hyper (a) heterogenously enhancing lumbar (L1- L4) extradural mass lesion with associated L3 vertebral body compression collapse
Discussion
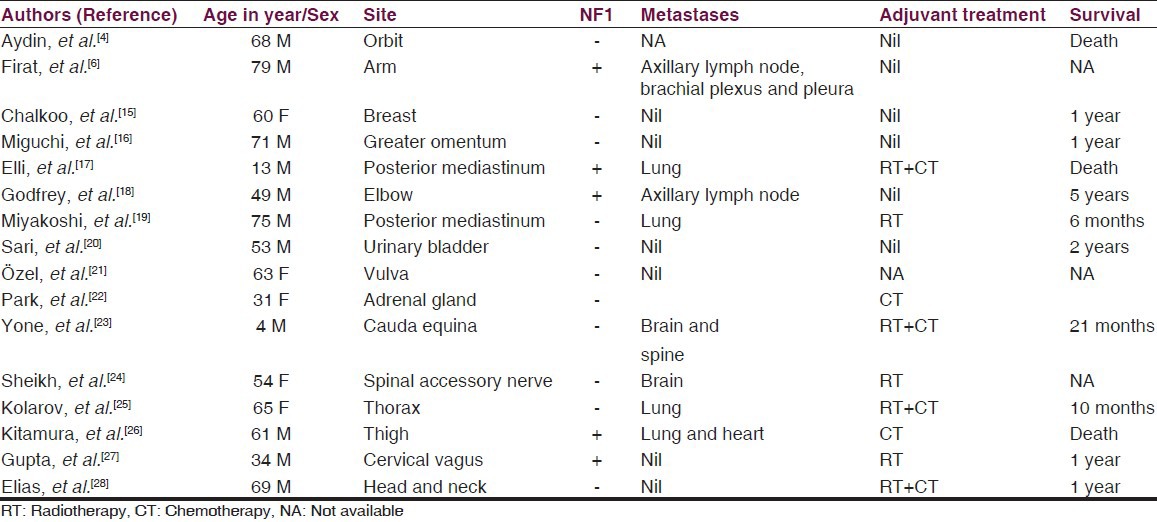
Malignant peripheral nerve sheath tumor (MPNST) is the preferred term for tumors originating from peripheral nerves or their sheaths and it has replaced the previous entities such as malignant schwannoma, malignant neurilemmoma and neurofibrosarcoma. They represent approximately 10% of all soft tissue sarcomas.[3] They may arise spontaneously, although in 5-42% of cases an association with neurofibromatosis (NF) Type 1 is known. MPNSTs commonly arise in adult patients ranging from 20 to 50 years of age. They originate from a major or minor peripheral nerve branch or its sheath. The common sites of origin include the extremities and trunk, usually sciatic nerve, brachial plexus and the sacral plexus. To our knowledge, few patients with a cranial or facial MPNST have been reported.[1,4] Likewise, cranial nerves are rarely affected, although tumors of the trigeminal and acoustic nerves have been reported.[5] Although rare, reports of MPNST arising at unusual sites have been documented by various authors [Table 1]. Firat et al.,[6] had reported an interesting case of arm MPNST with metastatic involvement of ipsilateral axillary lymph node, brachial plexus and pleura in a geriatric patient having multiple neurofibromas of different sizes almost covering his entire body, massively.
Table 1.
Published clinical studies and case reports of MPNSTs at unusual sites
Most cases are large, fleshy, often necrotic neoplasms averaging more than 5 cm in diameter.[7] MPNSTs are fusiform to globular in shape and vary from white and firm to yellow and soft, depending on the absence or presence of necrosis. Although the tumors usually appear well circumscribed, they are not truly encapsulated. The tumor in our case was greyish white, firm, with foci of hemorrhage and necrosis [Figures 1d and 4b].
The histological features of MPNSTs are those of a highly cellular, spindle-cell neoplasm resembling a soft-tissue sarcoma, but with differentiation toward elements of the nerve sheath, Schwann cell, and perineural cell.[8] Frequent mitoses and focal necrosis are typical. Rarely are heterologous mesenchymal or epithelial elements present. As in our case, MPNSTs can include heterologous mesenchymal and epithelial elements. Such atypical components show hypercellularity, an increased nuclear-to-cytoplasmic ratio, cytological atypia and increased mitotic activity.[9] The histological spectrum of MPNST is broad and the diagnosis rests on combination of some microscopic features, none of which is diagnostic by itself. S100 protein, the most widely used antibody for nerve sheath tumor, is positive only in 50% of MPNSTs.[10] Another diagnostic, Leu 7 immunoreactivity, is reported to be positive in 30-40% of the cases.[10] our case showed immunopositivity with S100 protein and was negative for other schwann cell markers. Data suggest that Leu-7 is an important marker of Schwann cell neoplasms, although it is not superior to S100 protein. Moreover, combined immunohistochemical evaluation of potential Schwann cell markers including Leu-7, MBP, GFAP and LN3 using commercially available antibodies offers no advantage over analysis of S100-protein immunoreactivity alone.[11]
Metastases occur in 39% of patients, lung being the most common metastatic site. The most important features adversely influencing prognosis are the presence of Von Recklinghausen's disease, a tumor larger than 5 cm and extent of resection.[12]
Radioimaging is helpful to know the exact site and extension of the tumor. Biopsy is necessary to diagnose an MPNST definitively. The differential diagnosis between benign schwannoma and neurosarcoma may be challenging: One must look for necrotic foci, the number of atypical mitoses, and an absence of differentiated cells. Tumors larger than 5 cm, histological grades II and III, an association with neurofibromatosis, and regional or distant metastases suggest an ominous prognosis.
The treatment of choice is surgery, but postoperative radio- and chemotherapy are part of adjunctive therapy.[13] Gross total resection of the tumor is the most important therapeutic goal. When radical tumor removal is not possible, excision combined with high-dose radiation therapy seems to be the best alternative treatment.[12] With the latest advances in molecular genetics, the target therapy for this tumor type is expected to be discovered.[14]
Conclusions
MPNST can arise in any unusual sites other than its common location at extremities. Existence of neurofibromatosis may not be present. Suspicion of MPNST should be raised in rapidly growing painless tumor in and around a nerve tissue. Complete surgical removal should be the goal of treatment with definitive histological diagnosis.[28]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.D’Agostino AN, Soule EH, Miller RH. Sarcomas of the peripheral nerves and somatic soft tissue associated with multiple neurofibromatosis (von Recklinghausen's disease) Cancer. 1963;16:1015–27. doi: 10.1002/1097-0142(196308)16:8<1015::aid-cncr2820160808>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Kchouk M, Rabet AM, Ghedas K, Nagi S, Douik M, Ben Romdhane K, et al. Extensive malignant schwannoma of the sciatic nerve. Contribution of imaging techniques. J Radiol. 1993;74:641–4. [PubMed] [Google Scholar]
- 3.Weiss SW, Goldblum JR. Malignant tumors of the peripheral nerves. In: Strauss M, Grey L, editors. Enzinger and Weiss's Soft Tissue Tumors. 4th ed. St. Louis: Mosby, Inc; 2001. pp. 1209–64. [Google Scholar]
- 4.Aydin MD, Yildirim U, Gundogdu C, Dursun O, Uysal HH, Ozdikici M. Malignant peripheral nerve sheath tumor of the orbit: Case report and literature review. Skull Base. 2004;14:109–13. doi: 10.1055/s-2004-828705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han DH, Kim DG, Chi JG, Park SH, Jung HW, Kim YG. Malignant triton tumor of the acoustic nerve. Case report. J Neurosurg. 1992;76:874–7. doi: 10.3171/jns.1992.76.5.0874. [DOI] [PubMed] [Google Scholar]
- 6.Firat C, Aytekin AH, Erbatur S. Metastatic malignant peripheral nerve sheath tumor in neurofibromatosis type 1: A geriatric patient report. Eur Rev Med Pharmacol Sci. 2012;16:1301–4. [PubMed] [Google Scholar]
- 7.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–21. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Chitale AR, Dickersin GR. Electron microscopy in the diagnosis of malignant schwannomas. A report of six cases. Cancer. 1983;51:1448–61. doi: 10.1002/1097-0142(19830415)51:8<1448::aid-cncr2820510819>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Enzinger FM, Weiss SW. 1st ed. St Louis, MO: CV Mosby; 1983. Soft Tissue Tumors; pp. 625–54. [Google Scholar]
- 10.Rosai J. Soft tissues. In: Ackerman R, editor. Surgical Pathology. 9th ed. Philadelphia, St Louis: Mosby; 2004. pp. 2237–371. [Google Scholar]
- 11.Johnson MD, Glick AD, Davis BW. Immunohistochemical evaluation of Leu-7, myelin basic-protein, S100-protein, glial-fibrillary acidic-protein, and LN3 immunoreactivity in nerve sheath tumors and sarcomas. Arch Pathol Lab Med. 1988;112:155–60. [PubMed] [Google Scholar]
- 12.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, IIlstrup DM. Malignant peripheral nerve sheath tumors. Cancer. 1986;57:2006–21. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Aguiar Vitacca S, Sarrazin D, Henry-Amar M, Spielmann M, Genin J, Bernheim A, et al. Neurosarcoma associated with Von Recklinghausen disease: Apropos of 25 cases observed at the Gustave Roussy Institute from 1967 to 1990. Bull Cancer. 1992;79:101–12. [PubMed] [Google Scholar]
- 14.Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62:1573–7. [PubMed] [Google Scholar]
- 15.Chalkoo M, Ahangar S, Laharwal AR, Patloo AM, Mohd A, Dar SA. Primary malignant peripheral nerve sheath tumor of the breast: A case report. Surg Sci. 2011;2:137–9. [Google Scholar]
- 16.Miguchi M, Takakura Y, Egi H, Hinoi T, Adachi T, Kawaguchi Y, et al. Malignant peripheral nerve sheath tumor arising from the greater omentum: Case report. World J Surg Oncol. 2011;9:33. doi: 10.1186/1477-7819-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elli M, Can B, Ceyhan M, Pinarli FG, Dagdemir AD, Ayyildiz HS, et al. Intrathoracic malignant peripheral nerve sheath tumor with angiosarcoma in a child with NF1. Tumori. 2007;93:641–4. doi: 10.1177/030089160709300625. [DOI] [PubMed] [Google Scholar]
- 18.Godfrey GJ, Farghaly H. Lymph node metastasis of malignant peripheral nerve sheath tumor in the absence of widespread disease five years after diagnosis: A rare finding. Int J Clin Exp Pathol. 2010;3:812–4. [PMC free article] [PubMed] [Google Scholar]
- 19.Miyakoshi N, Nishikawa Y, Shimada Y, Okada K, Yoshida M, Enomoto K, et al. Intraosseous malignant peripheral nerve sheath tumor with focal epithelioid differentiation of the thoracic spine. Neurol India. 2007;55:64–6. doi: 10.4103/0028-3886.30431. [DOI] [PubMed] [Google Scholar]
- 20.Sari A, Bal K, Tunakan M, Ozturk C. A case of sporadic malignant peripheral nerve sheath tumor of the urinary bladder with concomitant in situ urothelial carcinoma treated by transurethral resection. Indian J Pathol Microbiol. 2011;54:147–9. doi: 10.4103/0377-4929.77376. [DOI] [PubMed] [Google Scholar]
- 21.Özel E, Pesterel HE, Simsek T, Karavel FS, Trak B. Malignant peripheral nerve sheath tumor of the vulva: A case report. Turk J Canc. 2006;36:31–4. [Google Scholar]
- 22.Park JH, Ha SY, Cho HY. Malignant peripheral nerve sheath tumors of the bilateral adrenal glands: Are they metachronous primary tumors: A case report. Korean J Pathol. 2009;43:471–4. [Google Scholar]
- 23.Yone K, Ijiri K, Hayashi K, Yokouchi M, Takenouchi T, Manago K, et al. Primary malignant peripheral nerve sheath tumor of the cauda equine in a child case report. Spinal Cord. 2004;42:199–203. doi: 10.1038/sj.sc.3101567. [DOI] [PubMed] [Google Scholar]
- 24.Sheikh OA, Reaves A, Kralick FA, Brooks A, Musial RE, Gasperino J. Malignant nerve sheath tumor of the spinal accessory nerve: A unique presentation of a rare tumor. J Clin Neurol. 2012;8:75–8. doi: 10.3988/jcn.2012.8.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolarov V, Stanić J, Eri Ž, Zvezdin B, Kojičić M, Hromis S. Intrathoracic malignant peripheral nerve sheath tumor with poor outcome: A case report. Bosn J Basic Medl Sci. 2010;10:328–30. doi: 10.17305/bjbms.2010.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamura M, Wada N, Nagata S, Iizuka N, Jin YF, Tomoeda M, et al. Malignant peripheral nerve sheath tumor associated with neurofibromatosis type 1, with metastasis to the heart: A case report. Diagn Pathol. 2010;5:2. doi: 10.1186/1746-1596-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A, Bansal S, Bhagat S, Bahl A. Malignant peripheral nerve sheath tumor of the cervical vagus nerve in a neurofibromatosis type 1 patient: An unusual presentation. Online Journal of Health and Allied Sciences. 2010;9:1–3. [Google Scholar]
- 28.Elias MM, Balm AJ, Peterse JL, Keus RB, Hilgers FJ. Malignant schwannoma of the parapharyngeal space in von Recklinghausen's disease: A case report and review of the literature. J Laryngol Otol. 1993;107:848–52. doi: 10.1017/s0022215100124612. [DOI] [PubMed] [Google Scholar]


