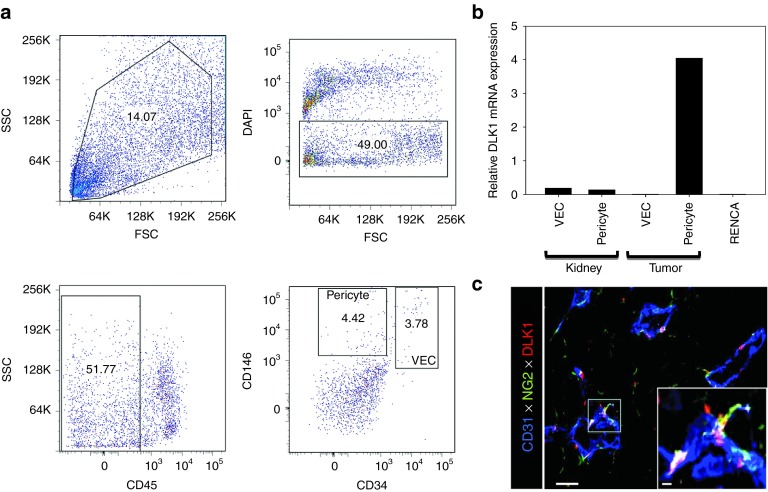
Figure 1.
DLK1 is differentially expressed by RENCA tumor-associated pericytes. The spontaneously arising renal cortical adenocarcinoma RENCA (106 tumor cells) was injected s.c. into female BALB/c mice and allowed to progress for 21 days after which animals were euthanized and tumors and normal kidneys harvested. (a) Tissues were processed into single-cell suspensions and sorted by flow cytometry based on forward versus side scatter profiles, DAPI exclusion (to reject dead cells), a CD45neg phenotype (i.e., non-leukocytic), and then selectively into CD146+CD34neg pericytes and CD146+CD34+ VEC populations based on published assignments of these cell lineage-restricted phenotypes.48,49 (b) mRNA was then isolated from flow-sorted pericytes and VEC, and analyzed for DLK1 transcript expression by real-time PCR. Relative mRNA expression was normalized to housekeeping HPRT1 mRNA expression. (c) Day 21 RENCA tumor tissue sections were analyzed for expression of CD31 (blue), NG2 (green), and DLK1 (red) by immunofluorescence microscopy. Metamorph quantitation (Materials and Methods) was performed on 10 high power field (HPF) of the fluorescent images, with 28.1 ± 4.4% of tumor-associated NG2+ pericytes coexpressing the DLK1 marker. The analysis also revealed that the majority (i.e., 58.9 ± 7.6%) of DLK1+ cells coexpressed the NG2 marker within the TME. All data are representative of three independent experiments performed. DAPI, 4′,6-diamidino-2-phenylindole; FSC, forward scatter; SSC, side scatter; TME, tumor microenvironment; VEC, vascular endothelial cell.