Figure 2.
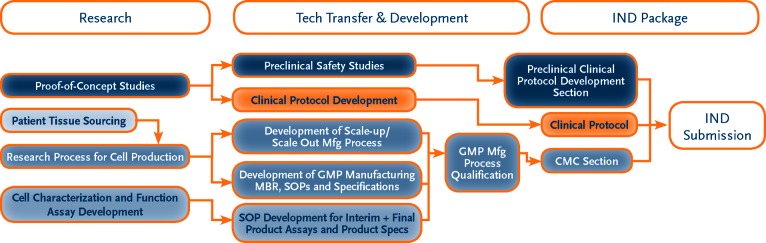
This flowchart represents the steps required for IND submission. Each developer should refer to the regulatory documents specific to its country and region for guidance prior to developing IND strategies. Abbreviations: CMC, Chemistry, Manufacturing, and Controls; GMP, Good Manufacturing Practice; IND, Investigational New Drug; MBR, Manufacturing Batch Record; Mfg, manufacturing; SOP, Standard Operating Procedure.
