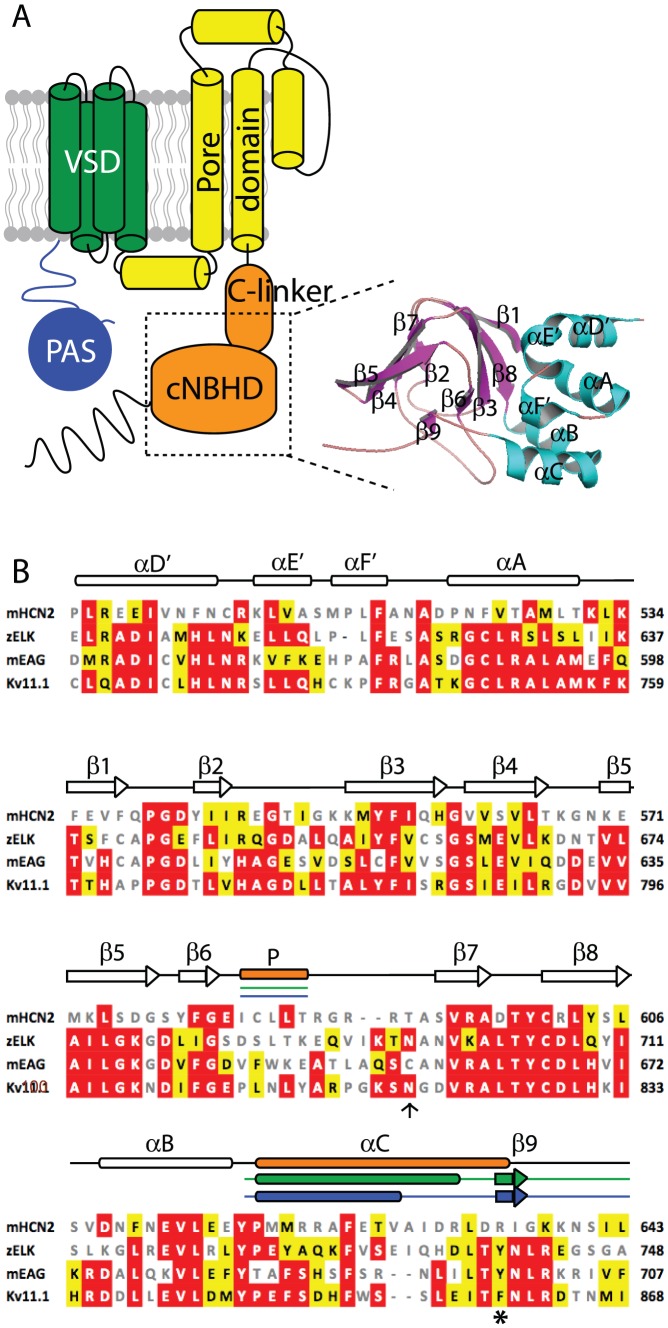
Figure 1. Topology of Kv11.1 channels and sequence analysis of cNBH domains.
(A) Topology of Kv11.1 channel showing the intracellular N-terminal PAS domain (blue), transmembrane voltage sensing domain (green), pore domain (yellow) and intracellular C-terminal C-linker and cNBH domains (orange). Inset shows the homology model of the cNBH domain of Kv11.1 generated based on the mEAG crystal structure [13]. (B) Sequence alignment of mHCN2, zELK, mEAG and human Kv11.1 extracted from a Clustalw alignment of the entire family of KCNHx/HCNx/CNGx ion channels. Sequences shown correspond to the dotted box region shown in panel A. Sequence similarity to the Kv11.1 are marked by white text/red box (identical) and black text/yellow box (similar). Non-conserved sequences are in grey. Clear rods and arrows represent the consensus α-helices and β-strands while filled rods and arrows indicate the differences with orange, green and blue representing mHCN2, zELK and mEAG, respectively. The hydrogen bond between asparagine (arrow) and tyrosine (asterisk) in zELK is not observed in the others.