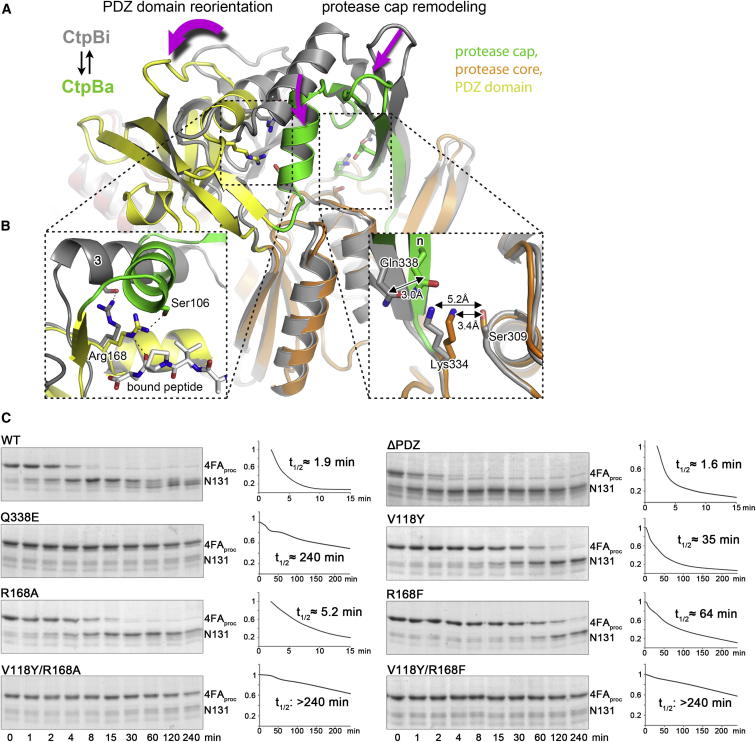
Figure 5.
Peptide Binding to the PDZ Domain Stabilizes the Active State
(A) CtpBa1 (colored according to domains) and CtpBi (gray) are shown with the protease core domains superimposed. Arrows indicate movements of the protease cap and the PDZ domain that accompany the transition from the resting to the active conformation.
(B) Zoomed-in windows illustrate key events underlying the switch in activity. Left: Aligned peptide-binding sites of the PDZ domain highlighting the catalytic role of Arg168. Upon substrate binding, the reoriented Arg168 can undergo additional interactions with the captured ligand (white) thereby stabilizing the active protease form. Dotted lines indicate hydrogen bonds. Right: Remodeling of the proteolytic site upon rearrangement of the protease cap. Distances between the functional groups of the catalytic residues are indicated and reveal the remodeling of the catalytic triad during the conformational switch.
(C) SDS-PAGE assays monitoring the degradation of 4FAproc by wild-type CtpB and various mutants (substrates and cleavage products are indicated). The right panels illustrate the time courses of substrate degradation yielding the indicated half-times of substrate cleavage. See also Figure S5.