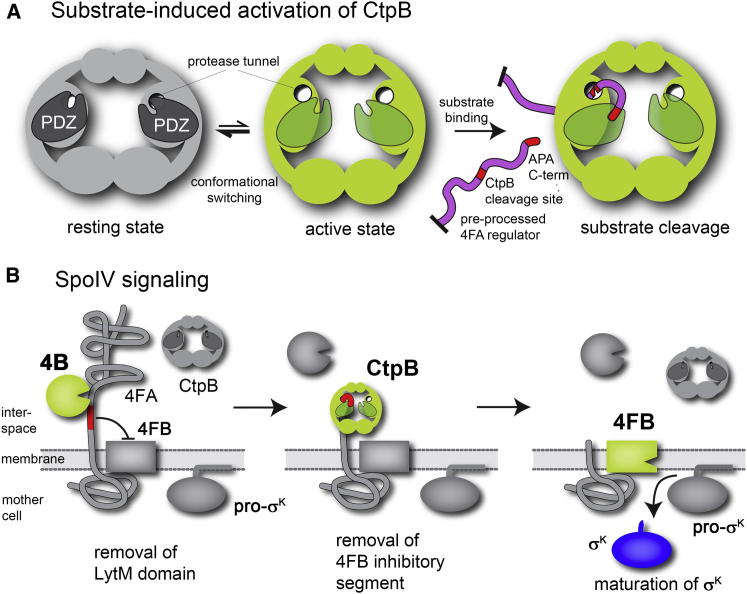
Figure 7.
Model for Cooperative Cleavage of 4FA by the Signaling Proteases 4B and CtpB
(A) Cartoon illustrating the allosteric regulation of CtpB. The CtpB protease can reversibly switch between active and resting conformations. When 4FAproc (magenta) binds to the PDZ domain, the activated CtpB removes the 4FA linker segment 131-154 protruding through the protease tunnel. CtpB cleavage site (Ala131) and 4B-processed C terminus (Ala152-Pro153-Ala154) are colored red.
(B) The SpoIV proteolytic cascade transmits the sporulation signal from forespore to mother cell. Our data indicate that the three participating proteases 4B, CtpB and 4FB act sequentially in the RIP pathway (respective active forms shown in green). Initially, the 4FA regulator is protected from CtpB cleavage by its folded C-terminal domain. Thus, CtpB activity strictly depends on the previous shedding of 4FA by the 4B protease. The truncated 4FA C terminus binds and activates CtpB, which in turn removes the inhibitory 4FA segment indicated in red. The unreleased I-CLiP membrane protease 4FB can now cleave the membrane-associated pro-σK sigma factor, releasing σK into the cytosol and allowing sporulation to proceed. See also Figure S6.