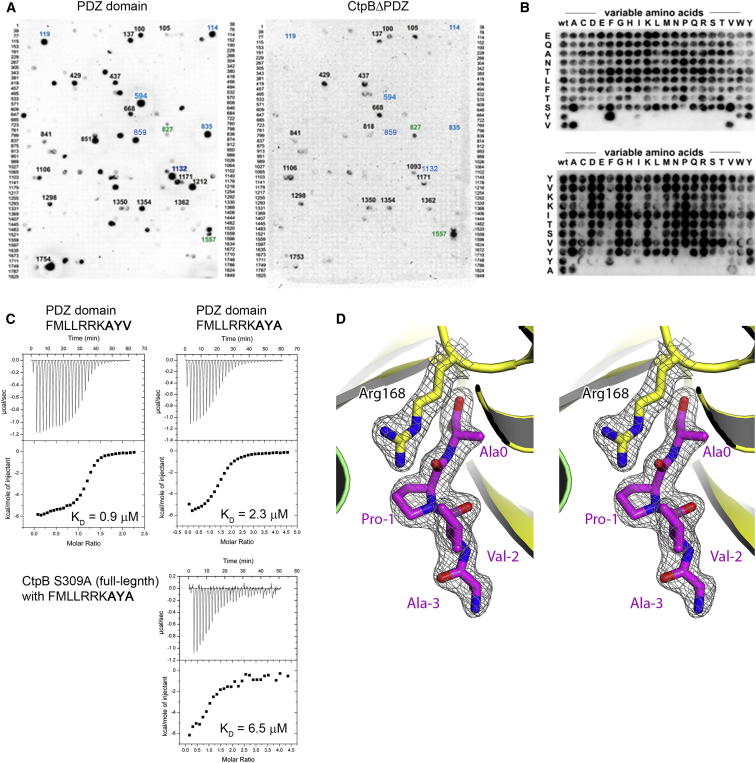
Figure S4.
Peptide-Binding Properties of the PDZ Domain, Related to Figure 4
(A) The C termini of B. subtilis proteins predicted to reside in the intermembrane space were synthesized on a cellulose membrane according to the method of inverted peptides. Spotted membranes were probed with either the PDZ domain of CtpB (left) or the ΔPDZ mutant (right). (B) Identified strong binding peptides were further analyzed by systematically substituting residues critical for binding to the PDZ domain of CtpB. (C) ITC analysis testing the interaction of the indicated peptides with the isolated PDZ domain and full-length CtpB (S309A). Raw data (top) and binding isotherm derived from the integrated heat (bottom) are shown. The determined KD dissociation constants are indicated below the binding curves. (D) Stereo view of the C-terminal Val-Pro-Ala-COOH motif that is accommodated in the PDZ domain of CtpB (S309A). Arg168 obtains an important role in tethering the backbone of the penultimate proline residue. The final model is overlaid with the omit 2Fo-Fc electron density (1.9 Å resolution, contoured at 1.2 σ) calculated without peptide ligand and Arg168.