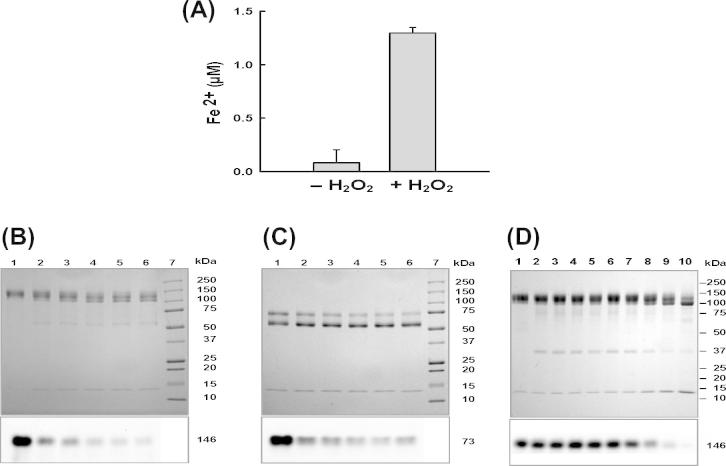
Fig. 4.
Structural consequences of myeloperoxidase incubated with hydrogen peroxide. (A) Detection of iron release from the heme prosthetic group. 1.5 μM MPO was reacted with 1.5 mM hydrogen peroxide in 50 mM sodium acetate buffer, pH 7.4, (column 2). Column 1 shows the result for untreated MPO. Ferrozine was used to measure the release of iron from the heme group (n = 3). (B, C) SDS–PAGE: Time course of MPO reacted with hydrogen peroxide. Top panels: 1.5 μM MPO was reacted with 1.5 mM of hydrogen peroxide in 50 mM phosphate buffer, pH 7.4, and 100 μM DTPA. 15 μL aliquots were taken at 5 min (lane 2), 10 min (lane 3), 25 min (lane 4), 45 min (lane 5) and 60 min (lane 6) and added to 20 μg/mL catalase to remove remaining hydrogen peroxide. Lane 1: negative control (15 μL of untreated MPO, no catalase added). The samples were run on a 8–20% resolving SDS–PAGE under non-reducing (B) and reducing (C) conditions. Bottom panels: SDS–PAGE run under the same conditions as shown in top panels, blotted onto a PVDF membrane and detection of the heme by chemiluminescence. (D) 1.5 μM MPO was incubated with hydrogen peroxide in 50 mM phosphate buffer, pH 7.4, containing 100 μM DTPA for 2 h at various hydrogen peroxide to MPO ratios; 1:1 (lane 2), 5:1 (lane 3), 10:1 (lane 4), 40:1 (lane 5), 100:1 (lane 6), 167:1 (lane 7), 500:1 (lane 8), 1000:1 (lane 9) and 2000:1 (lane 10). 15 μL aliquots were taken and resolved on a 8–20% SDS PAGE under non-reducing conditions. Lane 1: negative control (15 μL of untreated MPO). Bottom panel: SDS–PAGE run under the same conditions as shown in top panel, blotted onto a PVDF membrane and detection of the heme by chemiluminescence.