Abstract
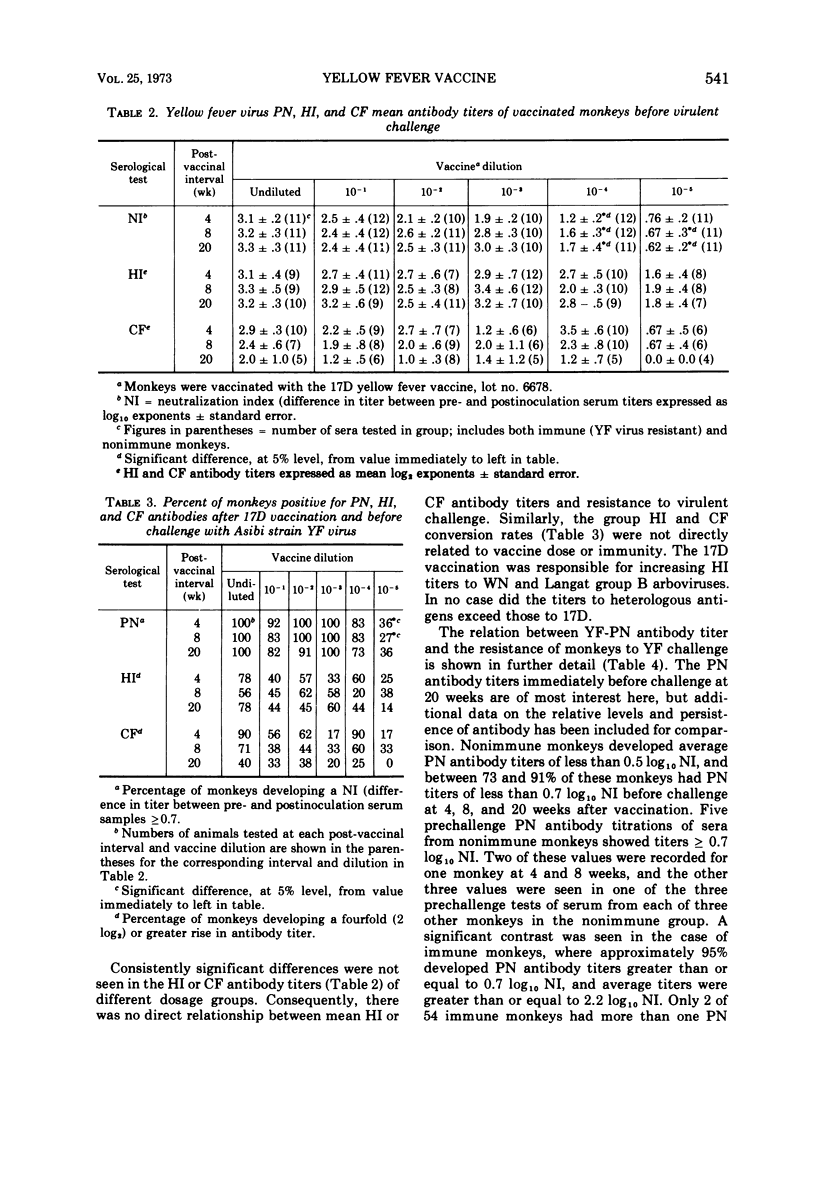
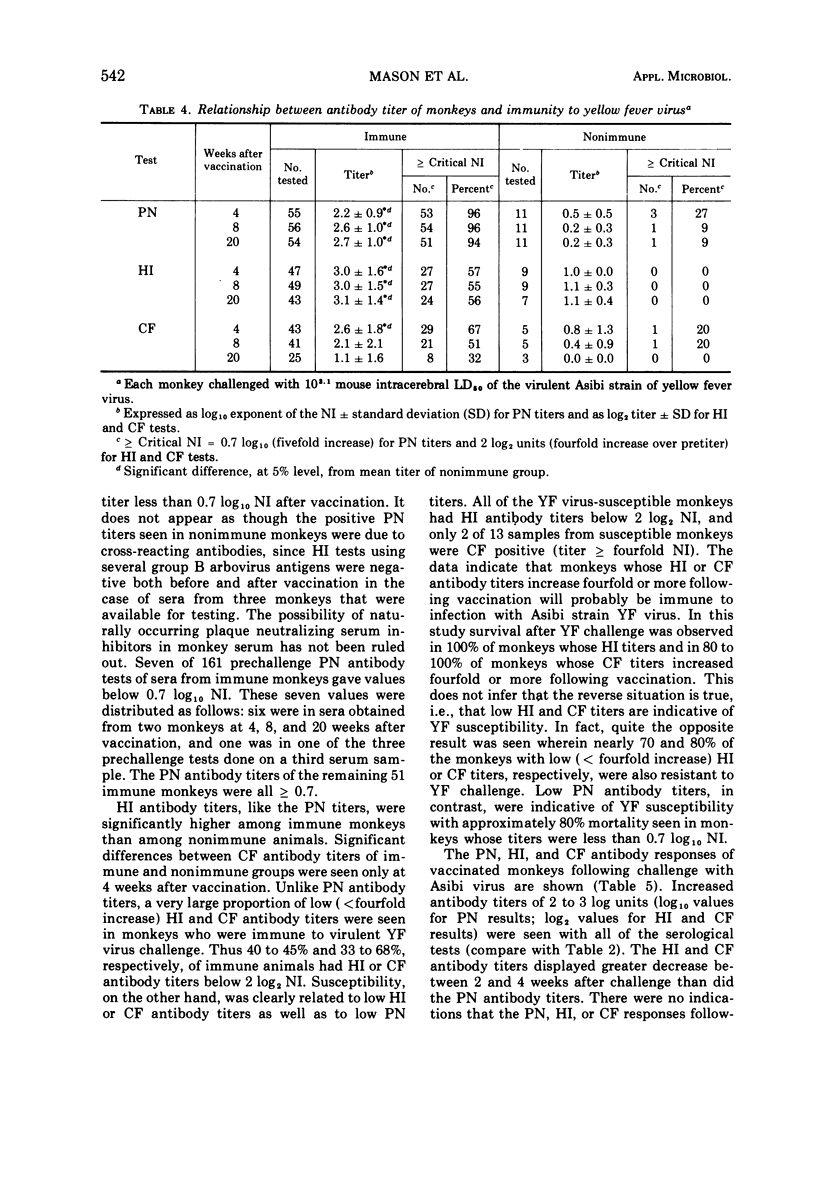
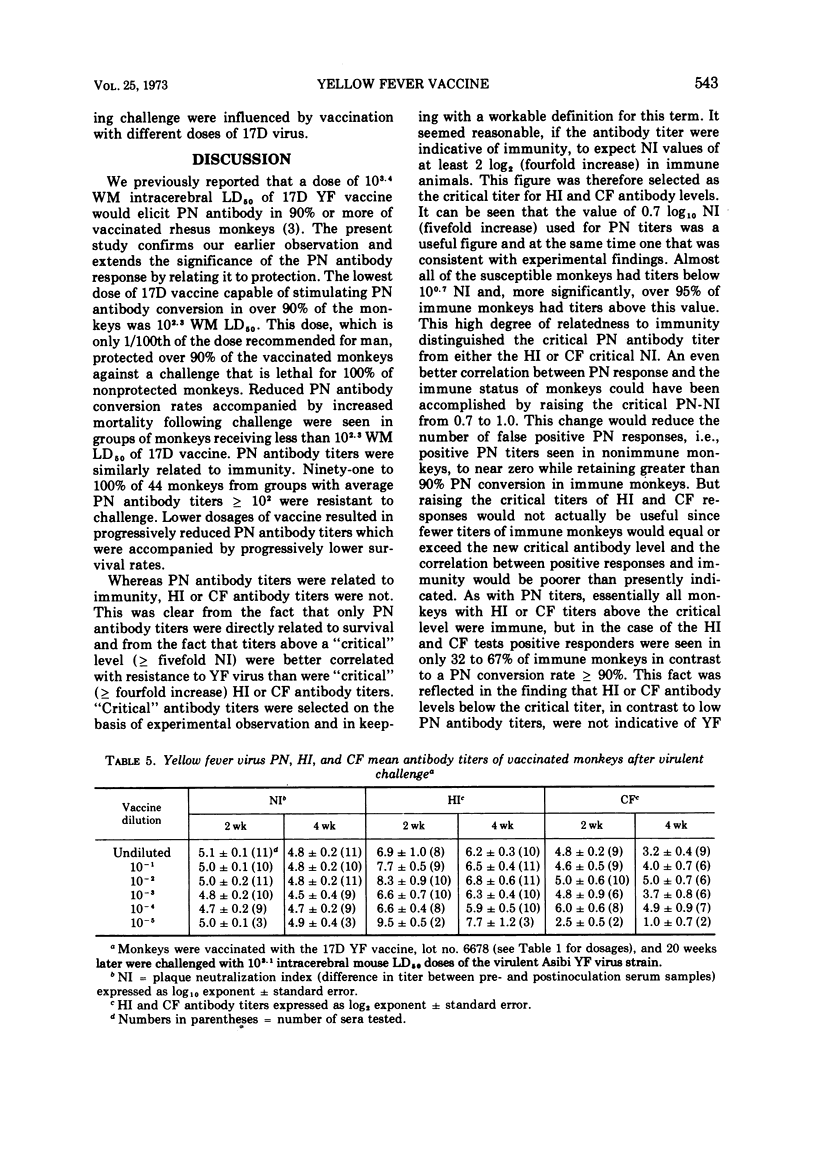
In rhesus monkeys a wide dosage range of 17D yellow fever (YF) vaccine extending to a level even below that recommended for vaccination of man elicited an immune response providing solid protection to challenge with virulent YF virus. Forty-three of 45 monkeys vaccinated with 102.3 or greater weanling mouse mean lethal doses of 17D vaccine were resistant to challenge 20 weeks later with virulent Asibi strain YF virus. Monkeys given graded doses of lesser amounts of vaccine were progressively more susceptible to challenge. With a vaccine dose ≥ 102.3 weanling mouse mean lethal doses, plaque neutralization (PN) seroconversion rates were 90% or greater, whereas hemagglutination-inhibiting (HI) and complement-fixing (CF) seroconversion rates were unrelated to vaccine dosage and were generally in the range of 20 to 80%. Ninety-six percent (51 of 54) of immune monkeys had PN titers ≥0.7 log10 (fivefold) neutralization index as compared to approximately 55 to 65% who showed HI or CF titers ≥2 log2 (fourfold) neutralization index. After challenge with Asibi strain YF virus, antibody titers of all three tests increaed equally. In rhesus monkeys PN antibody titers were well correlated with YF immunity, whereas HI and CF antibody titers were not.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Draper C. C. A yellow fever vaccine free from avian leucosis viruses. J Hyg (Lond) 1967 Dec;65(4):505–513. doi: 10.1017/s0022172400046040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROOT H. Serological reactions in Rhesus monkeys inoculated with the 17D strain of yellow fever virus. Bull World Health Organ. 1962;27:709–715. [PMC free article] [PubMed] [Google Scholar]
- Mason R. A., Tauraso N. M., Ginn R. K., O'Brien T. C., Trimmer R. W. Yellow fever vaccine. V. Antibody response in maonkeys inoculated with graded doses of the 17D vaccine. Appl Microbiol. 1972 May;23(5):908–913. doi: 10.1128/am.23.5.908-913.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S., Tauraso N. M. Yellow fever virus. I. Development and evaluation of a plaque neutralization test. Appl Microbiol. 1968 Nov;16(11):1770–1775. doi: 10.1128/am.16.11.1770-1775.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauraso N. M., Coultrip R. L., Legters L. J., Richman A. V., Rosenberg D. M., Savadge T. O., Shelokov A., Spector S. L., Trimmer R. W. Yellow fever vaccine. IV. Reactogenicity and antibody response in volunteers inoculated with a vaccine free from contaminating avian leukosis viruses. Proc Soc Exp Biol Med. 1972 Feb;139(2):439–446. doi: 10.3181/00379727-139-36161. [DOI] [PubMed] [Google Scholar]
- Tauraso N. M., Spector S. L., Jahnes W. G., Shelokov A. Yellow fever vaccine. I. Development of a vaccine seed free from contaminating avian leukosis viruses. Proc Soc Exp Biol Med. 1968 Apr;127(4):1116–1120. doi: 10.3181/00379727-127-32885. [DOI] [PubMed] [Google Scholar]


