Significance
This study defines a functional role for βIV-spectrin in pancreas, expands the pathways for calcium/calmodulin-dependent protein kinase II (CaMKII) local control in excitable cells, and identifies a mechanism for CaMKII-dependent regulation of KATP channels.
Keywords: trafficking, local regulation
Abstract
Identified over a dozen years ago in the brain and pancreatic islet, βIV-spectrin is critical for the local organization of protein complexes throughout the nervous system. βIV-Spectrin targets ion channels and adapter proteins to axon initial segments and nodes of Ranvier in neurons, and βIV-spectrin dysfunction underlies ataxia and early death in mice. Despite advances in βIV-spectrin research in the nervous system, its role in pancreatic islet biology is unknown. Here, we report that βIV-spectrin serves as a multifunctional structural and signaling platform in the pancreatic islet. We report that βIV-spectrin directly associates with and targets the calcium/calmodulin-dependent protein kinase II (CaMKII) in pancreatic islets. In parallel, βIV-spectrin targets ankyrin-B and the ATP-sensitive potassium channel. Consistent with these findings, βIV-spectrin mutant mice lacking CaMKII- or ankyrin-binding motifs display selective loss of expression and targeting of key protein components, including CaMKIIδ. βIV-Spectrin–targeted CaMKII directly phosphorylates the inwardly-rectifying potassium channel, Kir6.2 (alpha subunit of KATP channel complex), and we identify the specific residue, Kir6.2 T224, responsible for CaMKII-dependent regulation of KATP channel function. CaMKII-dependent phosphorylation alters channel regulation resulting in KATP channel inhibition, a cellular phenotype consistent with aberrant insulin regulation. Finally, we demonstrate aberrant KATP channel phosphorylation in βIV-spectrin mutant mice. In summary, our findings establish a broader role for βIV-spectrin in regulation of cell membrane excitability in the pancreatic islet, define the pathway for CaMKII local control in pancreatic beta cells, and identify the mechanism for CaMKII-dependent regulation of KATP channels.
The coordinate expression, localization, and regulation of ion channels are critical for excitable cell biology, namely, neuronal transmission, cardiac excitation–contraction coupling, endocrine/exocrine secretory regulation, and skeletal muscle function. The spectrin family of polypeptides, originally discovered as a critical component of the erythrocyte plasma membrane over 30 y ago, is now known to play important structural roles in both nonexcitable and excitable cells (1, 2). Spectrin alpha (α) and beta (β) subunits form antiparallel dimers that self-associate to form tetramers, creating a submembranous scaffolding network anchored to actin filaments via β-spectrin (2). Whereas two genes encode α-spectrin polypeptides (αI-, αII-spectrin), spectrin family diversity is created through assembly of α-spectrin products with five β-spectrin genes (encoding βI–βV-spectrin) (2). Over a dozen years ago, βIV-spectrin was identified in brain and pancreatic islets (3). Since these findings, a host of studies have identified βIV-spectrin as a critical player for defining local membrane domains in the nervous system (4–6). Notably, βIV-spectrin complexes serve essential roles in targeting ankyrin and associated membrane proteins, to axon initial segments and nodes of Ranvier. Dysfunction in βIV-spectrin leads to aberrant membrane protein targeting, cell and animal phenotypes, and early death (6, 7). Despite these striking findings in brain, the role of βIV-spectrin in pancreatic islets is unknown.
Here, we define a role for βIV-spectrin in regulating membrane excitability in islets. βIV-spectrin directly associates with ankyrin-B and Ca2+/calmodulin-dependent protein kinase II (CaMKII). We define the structural requirements for these interactions and show that βIV-spectrin associates with additional islet proteins, including actin and the ATP sensitive potassium channel (KATP channel). Notably, βIV-spectrin mutant mice that lack ankyrin- or CaMKII-binding activity display coordinate loss of ankyrin, KATP channel, and CaMKII expression and targeting. Finally, we demonstrate that βIV-spectrin is critical for CaMKII-dependent regulation of the KATP channel through Kir6.2 residue T224 and show that Kir6.2 T224 phosphorylation is altered in βIV-spectrin mutant mice. In summary, our findings provide initial insight into the role of βIV-spectrin in the expression, targeting, and regulation of critical membrane and signaling proteins in islets.
Results
βIV-Spectrin Associates with CaMKII in Pancreas.
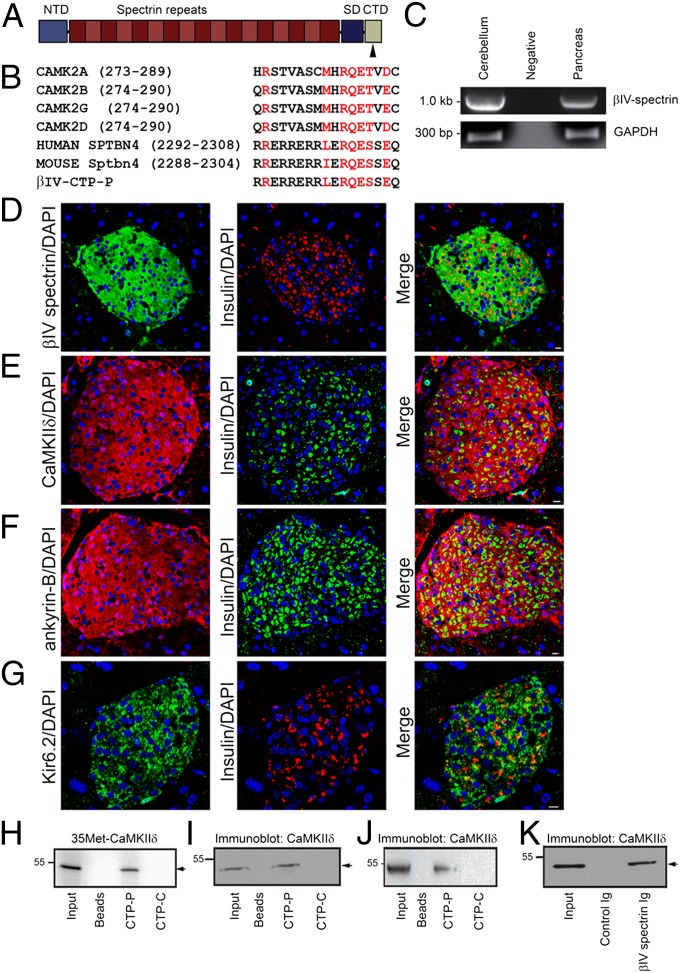
βIV-Spectrin contains an N-terminal actin-binding domain, a series of 17 spectrin repeats, and a C-terminal domain (Fig. 1A). The C-terminal domain contains a conserved motif harboring high homology to the autoregulatory domain of Ca2+/calmodulin-dependent protein kinase II (CaMKII), a site necessary for CaMKII intramolecular interaction (Fig. 1B) (8). This motif is present in a handful of CaMKII-associated proteins including the L-type Ca2+ channel beta subunit (β2a) and the NR2B receptor (9). Given the lack of data on βIV-spectrin function in the islet, we hypothesized that βIV-spectrin may function in the pancreas to control CaMKII targeting and expression, similar to observed roles in myocytes (10).
Fig. 1.
βIV-Spectrin is a CaMKII-binding partner in the pancreatic islet. (A and B) βIV-Spectrin has an N-terminal actin-binding domain (NTD), 17 spectrin repeats, and a specific/C-terminal domain (SD/CTD). The CaMKII-binding site is denoted by an arrow that is homologous to the CaMKII autoregulatory domain motif in all CaMKII genes and conserved across species (red font). (C) βIV-Spectrin mRNA expression in mouse cerebellum and pancreas. Immunostaining of mouse islets for (D) βIV-spectrin, (E) CaMKIIδ, (F) AnkB, and (G) Kir6.2. Sections were colabeled to denote nuclei (DAPI) and beta cells (insulin Ig). (Scale bar, 10 μm.) (H) CTP-P (peptide homologous to the CaMKII-binding motif of βIV-spectrin) directly binds radiolabeled CaMKIIδ (35Met-CaMKIIδ) in pull-down experiments. No appreciable binding was observed with beads or control CTP-C (control scrambled) peptide. (I and J) CTP-P peptide associates with endogenous CaMKIIδ from whole pancreas (I) and pancreatic islet lysate (J) in pull-down experiments. We observed no binding with beads alone or the control CTP-C peptide. (K) βIV-Spectrin Ig immunoprecipitates endogenous pancreatic CaMKIIδ, with no binding observed with control Ig or beads alone.
We confirmed βIV-spectrin mRNA and protein expression in the murine pancreas. Indeed, consistent with the only published data on pancreatic βIV-spectrin (3), βIV-spectrin is found in pancreas (Fig. 1C) and expressed in islets by immunoblot and immunostaining (Fig. 1D and Fig. 2A). In contrast, we observed minimal βIV-spectrin immunostaining in exocrine acinar cells surrounding the islet (Fig. 1D). Like βIV-spectrin, we observed expression of the CaMKII isoform δ within the murine islet (Fig. 1 E and J).
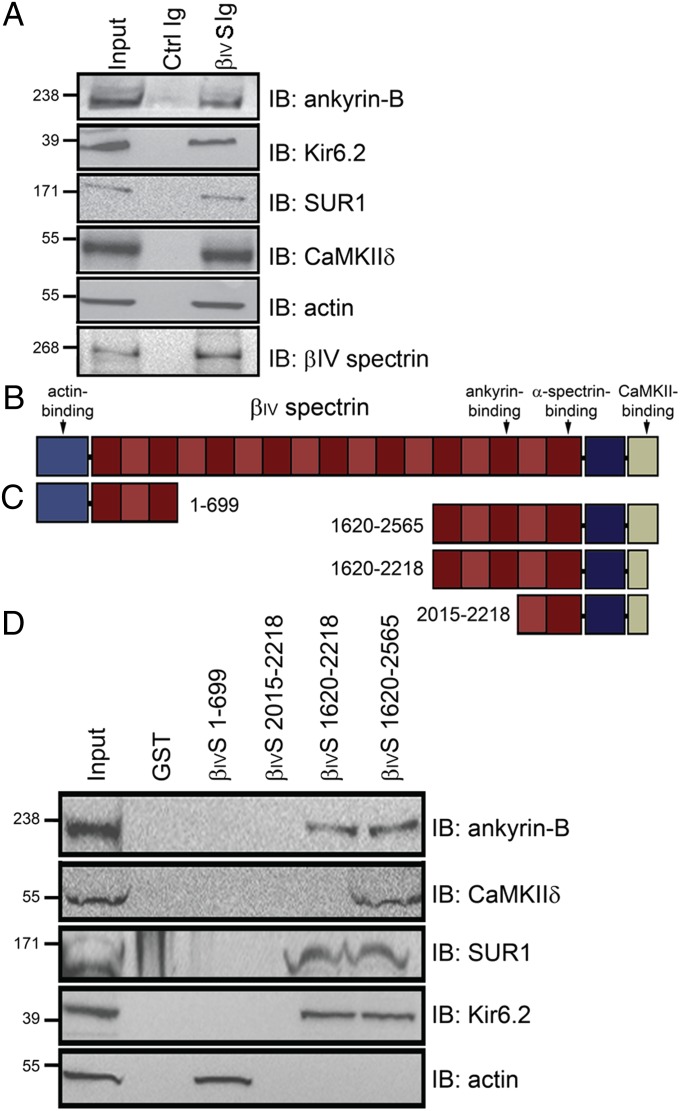
Fig. 2.
βIV-Spectrin–associated proteins in pancreatic islets. (A) βIV-Spectrin associates with AnkB, Kir6.2, SUR1, CaMKIIδ, and actin from pancreatic islet lysate by coimmunoprecipitation. (B and C) Structure of βIV-spectrin and βIV-spectrin GST-fusion constructs used in pull-down experiments from detergent-soluble pancreas lysates. (D) Whereas CaMKIIδ associates only with βIV-spectrin fusion constructs containing the distal C-terminal CaMKII binding motif in islet lysates, βIV-spectrin–dependent association with AnkB, Kir6.2, and SUR1 requires residues 1620–2218 (includes 15th spectrin repeat). βIV-Spectrin 1–699 was the only construct to copurify with actin from islet lysates. We observed no interaction of either CaMKIIδ or AnkB with N-terminal GST–βIV-spectrin fusion construct.
We tested a potential βIV-spectrin/CaMKII interaction in pancreas using radiolabeled protein and purified mouse pancreatic islet lysate. βIV-Spectrin directly associates with CaMKIIδ in vitro, determined using a peptide that corresponds to the putative CaMKII-binding motif in the βIV-spectrin C-terminal domain (CTP-P). CTP-P, but not beads alone nor CTP-C (βIV-spectrin C-terminal scrambled control peptide), bound radiolabeled CaMKIIδ (Fig. 1H) and endogenous CaMKIIδ from isolated pancreatic islets or whole pancreas (Fig. 1 I and J). Furthermore, βIV-spectrin Ig, but neither control Ig nor beads alone, was able to immunoprecipitate with CaMKIIδ from pancreas and pancreatic islet cell lysates (Figs. 1K and 2A). Together, these data support a βIV-spectrin/CaMKIIδ interaction in pancreatic islets via a C-terminal domain in βIV-spectrin.
Identification of βIV-Spectrin– and Ankyrin-B–Associated Proteins in Islets.
β-Spectrin polypeptides associate with ankyrin proteins through a conserved binding motif in the β-spectrin 15th spectrin repeat (11). Ankyrin-B (AnkB) is present within the islet and necessary for the targeting and expression of the ATP-sensitive potassium channel (KATP) (12) and inositol 1,4,5 trisphosphate (IP3) receptor (13). Human mutations in Kir6.2 (KATP channel α-subunit) that block binding with AnkB are associated with neonatal diabetes mellitus (12, 14), although these variants may also affect KATP Kir6.2/SUR1 subunit coupling (15). Consistent with βIV-spectrin localization, AnkB and Kir6.2 are highly expressed in the islet where the KATP channel regulates cellular excitability and insulin secretion (Fig. 1 F and G). Moreover, AnkB and βIV-spectrin associate from purified islet lysates by coimmunoprecipitation (Fig. 2A). In addition to ankyrin-B, we also observed CaMKIIδ, Kir6.2, and SUR1 (KATP channel subunit) in βIV-spectrin coimmunoprecipitation experiments (Fig. 2A).
Defining the Structural Requirements for βIV-Spectrin Binding for CaMKII and AnkB.
We constructed a series of βIV-spectrin GST-fusion proteins containing key regions of the molecule to define the structural requirements for βIV-spectrin association with CaMKII and AnkB (Fig. 2 B and C). Consistent with peptide pull-down experiments, a βIV-spectrin fusion protein containing the full C terminus (residues 1620–2565) associated with CaMKIIδ from islets (Fig. 2D and Fig. S1). In contrast, fusion proteins lacking the putative CaMKII-association motif (residues 1620–2218 and 2015–2218) did not bind islet CaMKIIδ (Fig. 2D). We observed no CaMKII-binding activity for a control βIV-spectrin N-terminal fusion protein (residues 1–699; Fig. 2D). On the other hand, C-terminal βIV-spectrin GST-fusion proteins containing residues 1620–2218 and 1620–2565 displayed robust AnkB-binding activity, whereas truncation of the fusion proximal to spectrin repeat 16 (residues 2015–2218) resulted in loss of AnkB-binding activity (Fig. 2D). We previously demonstrated that AnkB directly associates with Kir6.2 (12, 16) and AnkB/SUR1 interactions require Kir6.2 (Fig. S2). Consistent with these data, C-terminal βIV-spectrin 1620–2218 and 1620–2565 displayed binding activity for Kir6.2 and SUR1, but residues 2015–2218 lacked ability to associate with Kir6.2 or SUR1 (Fig. 2D). We observed no binding of the βIV-spectrin N terminus to CaMKII, AnkB, Kir6.2, or SUR1; however, as expected, this domain associated with actin (Fig. 2D). Our results support a model where βIV-spectrin associates with actin, AnkB, and CaMKIIδ through its N terminus, central spectrin repeat domain, and C terminus, respectively.
βIV-Spectrin Mutant Mice Display Select Loss of CaMKII and Ankyrin Binding.
Nearly 60 y ago, a spontaneous line of “quivering” (qv) mice was identified that displayed severe ataxia, hind-limb paralysis, deafness, and tremor (7). Subsequent studies identified specific mutations across the murine βIV-spectrin gene, resulting in the qv mouse phenotypes. These in vivo models have proved critical in defining the roles of βIV-spectrin function in physiology and disease (7). To test the role of βIV-spectrin in islet channel and signaling molecule expression and targeting, we used qv mouse lines harboring mutations that selectively alter AnkB (qv4J) and CaMKII association (qv3J; Fig. S3A). We coimmunoprecipitated CaMKIIδ, AnkB, Kir6.2, SUR1, and actin with βIV-spectrin from wild-type (WT) mouse islet lysates (Fig. S3B). Likewise, parallel AnkB Ig coimmunoprecipitations revealed βIV-spectrin, CaMKIIδ, Kir6.2, and SUR1 as AnkB-associated proteins (Fig. S3C). We observed loss of CaMKIIδ association, but not AnkB, Kir6.2, or SUR1, with βIV-spectrin in islet lysates from the qv3J mouse lacking spectrin/CaMKII interaction (Fig. S3 B and C); however, as noted in the next section, CaMKII protein levels are decreased in qv3J and qv4J islets, so coimmunoprecipitation inputs were adjusted to compensate for differences in protein expression (SI Materials and Methods). Additionally, CaMKIIδ immunoprecipitated with AnkB Ig in WT, but not qv3J islets (Fig. S3C). In qv4J mice lacking the ankyrin-binding domain, we observed loss of βIV-spectrin association with AnkB, Kir6.2, and SUR1, as well as inability of AnkB to associate with CaMKIIδ (Fig. S3 B and C). Finally, AnkB interactions with Kir6.2 and SUR1 were independent of βIV-spectrin or actin as AnkB Ig coimmunoprecipitated both Kir6.2 and SUR1 in qv4J islet lysates (Fig. S3C).
βIV-Spectrin Is Required for CaMKII Expression and Localization.
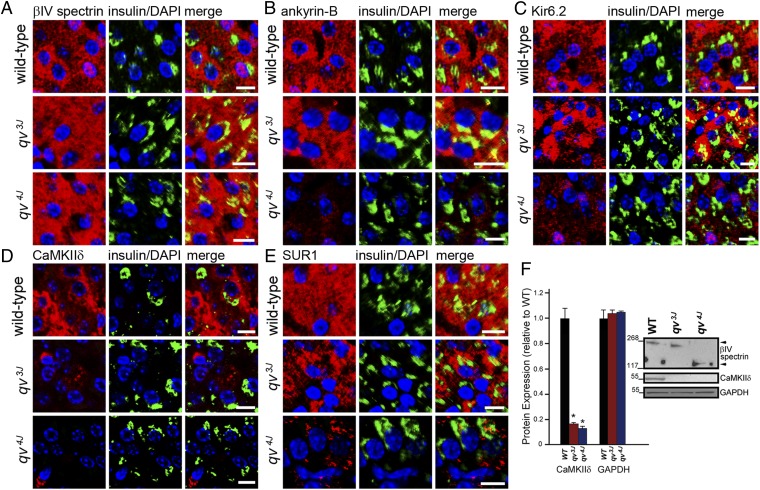
Based on our data, we hypothesized that βIV-spectrin regulates CaMKII posttranslational expression and targeting. We assessed the requirement of βIV-spectrin for CaMKII expression and targeting in WT, qv3J, and qv4J beta cells. As expected, βIV-spectrin was expressed throughout the cytosol of WT, qv3J, and qv4J beta cells (Fig. 3A). AnkB expression was present within both WT and qv3J beta cells, but consistent with the qv4J mutation lacking ankyrin-binding activity, we observed reduced and diffuse staining of AnkB in qv4J beta cells (Fig. 3B). In parallel with its dependence on AnkB for targeting, Kir6.2 (and SUR1) expression (expressed throughout cytosol) was significantly reduced in qv4J beta cells compared with WT, but not in qv3J islets (Fig. 3 C and E). Finally we observed loss of CaMKIIδ expression in both qv3J and qv4J beta cells (Fig. 3D). Immunoblots confirmed significantly reduced CaMKIIδ expression in islet lysates from both qv3J and qv4J models (Fig. 3F). βIV-spectrin may regulate the posttranscriptional stability of CaMKIIδ, as CaMKIIδ mRNA levels were unchanged between WT, qv3J, and qv4J mouse islets (Fig. S4). Alternatively, other cell or molecular mechanisms such as reduced CaMKII translation may underlie reduced expression of CaMKII in qv3J and qv4J islets.
Fig. 3.
βIV-Spectrin is required for CaMKIIδ, AnkB, Kir6.2, and SUR1 expression in beta cells. (A) WT, qv3J, and qv4J beta cells express βIV-spectrin [red; colabeled with DAPI (blue) and insulin (green)]. (B, C, and E) Whereas expressed in WT and qv3J beta cells, AnkB, Kir6.2, and SUR1 expression is decreased in qv4J islets. (D) Whereas concentrated in WT beta cells, CaMKIIδ expression is reduced in the qv3J and qv4J mouse beta cells. (Scale bar, 10 μm.) (F) CaMKIIδ protein levels are significantly decreased in qv3J and qv4J islet lysates (n = 4 mice, P < 0.05), whereas CaMKIIδ mRNA levels are unchanged (Fig. S4).
CaMKII Regulates KATP Channel Function.
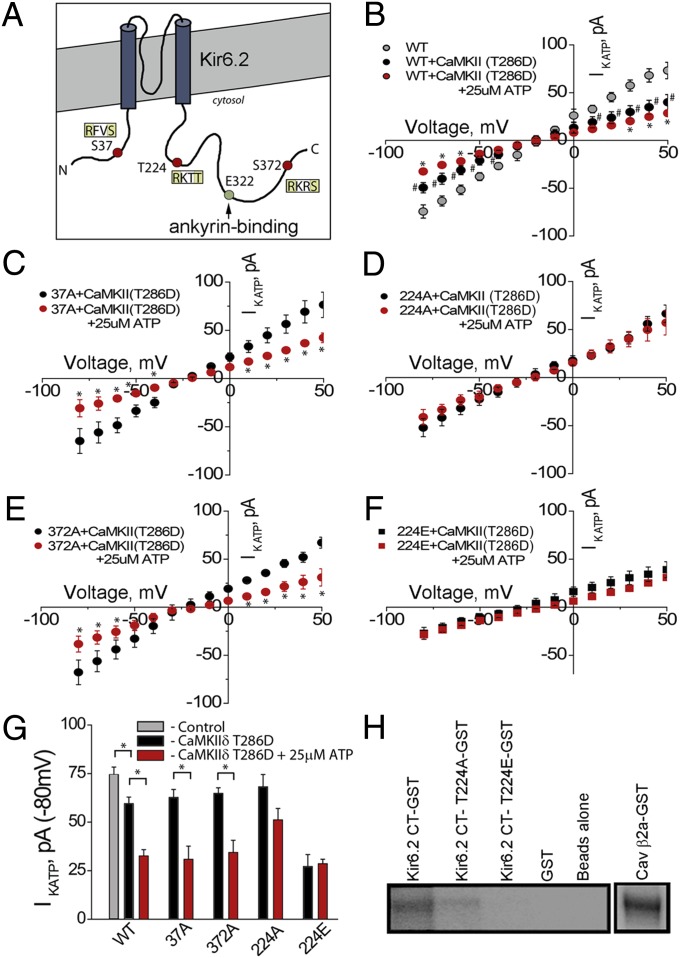
CaMKII activity has been linked with regulation of KATP channel function, albeit indirectly (17). We hypothesized that CaMKII may directly phosphorylate KATP channels via Kir6.2. We analyzed the primary amino acid sequence of Kir6.2 for putative CaMKII phosphorylation sites (using consensus sequence [RXXS/T]). Three putative CaMKII phosphorylation sites were identified: S37, T224, and S372 (Fig. 4A) and site-directed mutagenesis was used to substitute an alanine at each site. To determine if these mutants altered Kir6.2 function, we analyzed the activity of WT Kir6.2 (with SUR1) and mutant channels in COSm6 cultured cells that lack significant IKATP. Transfected cells were evaluated in the absence and presence of a constitutively active CaMKII (CaMKIIδ T286D). Cells expressing WT Kir6.2 showed significant decrease in IKATP when expressed with active CaMKII, as did Kir6.2 S37A and Kir6.2 S372A (Fig. 4 B, C, and E). However, cells transfected with Kir6.2 T224A showed no significant decrease in IKATP in the presence of active CaMKII (Fig. 4 D and G). Notably, when T224 was substituted with a glutamic acid to create a phosphomimetic (Kir6.2 T224E), we observed a significant decrease in KATP channel current at baseline (compared with WT Kir6.2), even in the absence of CaMKIIδ overexpression (Fig. 4 F and G).
Fig. 4.
CaMKII regulates KATP channel function. (A) Kir6.2 with AnkB-binding site denoted at Kir6.2 E322. Image denotes three putative CaMKII-phosphorylation sites: S37, T224, and S372. (B) COSm6 cells transfected with Kir6.2/CaMKII T286D demonstrate a significant reduction in IKATP, compared with control, ±ATP (25 μM). Likewise, we observed significant reductions in IKATP in cells transfected with CaMKII T286D and Kir6.2 S37A (C) and Kir6.2 S372A (E), ±25 μM ATP. We observed no significant change in IKATP in cells transfected with CaMKII T286D/Kir6.2 T224A, ±25 μM ATP (D). (F) Finally, transfection with CaMKII T286D/Kir6.2 T224E (phosphomimetic) demonstrates a significant reduction in IKATP compared with control, but no significant difference with and without ATP administration. (G) Summary of functional IKATP data (n > 7 cells per treatment; P < 0.05). (H) Purified and immobilized Kir6.2, Kir6.2 T224A, and Kir6.2 T224E were assessed for CaMKII-mediated phosphorylation in vitro. Control (β2a) and Kir6.2 showed robust CaMKII-mediated phosphorylation. There was significantly reduced phosphorylation of Kir6.2 T224A and Kir6.2 T224E.
To further analyze this site as a potential CaMKII target, we performed in vitro phosphorylation assays on WT and Kir6.2 T224 mutant proteins. Whereas β2a, a known CaMKII phosphorylation target and WT Kir6.2 were phosphorylated in vitro by CaMKII, Kir6.2-T224A and Kir6.2-T224E showed no significant CaMKII-mediated phosphorylation (Fig. 4H). Together, these data strongly support the role of CaMKII direct phosphorylation, demonstrate that this phosphorylation is at least in part direct, and implicate Kir6.2 T224 as a CaMKII target site in this pathway.
CaMKII Regulates Kir6.2 by Phosphorylation of Kir6.2 T224.
We created an affinity-purified phospho-specific antibody against Kir6.2 pT224 to evaluate the role of the βIV-spectrin/CaMKII pathway for KATP regulation in pancreas. The specificity of the antibody for the Kir6.2 pT224 site was confirmed by immunoblots that detected GFP-Kir6.2 from transfected cells, but not Kir6.2 T224A or Kir6.2 T224E (Fig. S5).
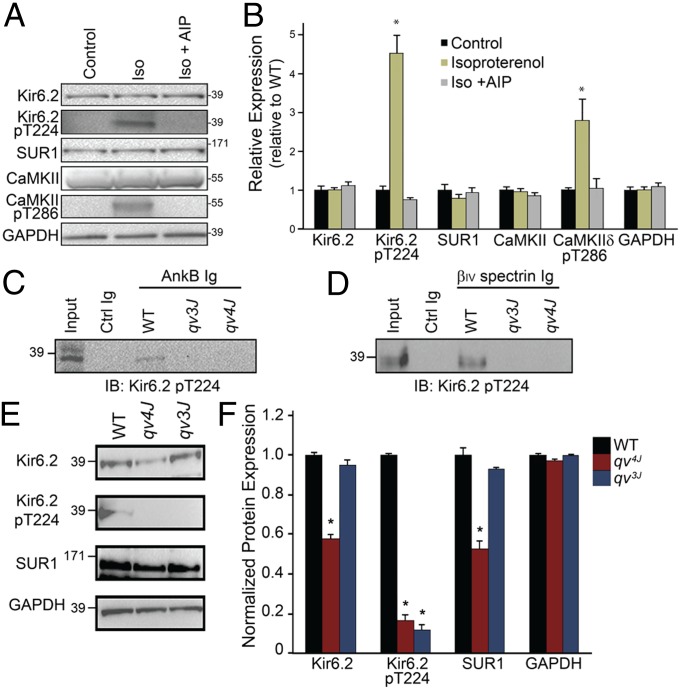
We evaluated Kir6.2 pT224 Ig in INS-1 cells, a rat insulinoma cell line commonly used to evaluate Kir6.2 and insulin secretion. Cells were analyzed for Kir6.2 pT224 ± isoproterenol (1 μM) to stimulate CaMKII activity. Notably, we observed nearly a fourfold increase in Kir6.2 pT224 [and twofold increase in activated CaMKII (CaMKII pT286)] in isoproterenol (Iso)-treated cells compared with untreated cells, whereas we observed no difference in total Kir6.2 or SUR1 levels (Fig. 5 A and B). To determine whether this activation required CaMKII, we performed parallel experiments in the presence of the CaMKII inhibitor, autocamtide-2–related inhibitory peptide (AIP). In support of a role of CaMKII in regulation of Kir6.2 pT224, AIP blocked both CaMKII pT286 and Kir6.2 pT224 activation (Fig. 5 A and B).
Fig. 5.
CaMKII regulates Kir6.2 by direct phosphorylation of Kir6.2 T224. (A and B) INS-1 display an increase in Kir6.2 pT224 and CaMKII pT286 expression when stimulated with isoproterenol (n = 3 per treatment, P < 0.05). CaMKII inhibitor AIP (Iso + AIP) blocked CaMKII T286 and Kir6.2 T224 phosphorylation (n = 3 per treatment, NS vs. control). (C and D) AnkB and βIV-spectrin associate with Kir6.2 pT224 in WT, but not qv3J or qv4J pancreatic islet lysates. (E and F) Kir6.2 pT224 levels are decreased in qv3J and qv4J pancreatic islet lysates. Expression presented relative to GAPDH expression (n = 3 per treatment, P < 0.05). In F, expression is normalized to GAPDH and compared with WT levels.
Consistent with (i) a role for βIV-spectrin in CaMKII regulation and (ii) a role for CaMKII in the direct regulation of Kir6.2 in vivo T224, Kir6.2 pT224 associated with βIV-spectrin and AnkB in coimmunoprecipitation experiments from WT, but not qv3J or qv4J, pancreatic islet lysates (Fig. 5 C and D). Further, we observed decreased levels of Kir6.2 pT224 in qv3J and qv4J pancreatic islet lysates (Fig. 5 E and F) compared with WT islets. In agreement with immunostaining data, we observed decreased Kir6.2 and SUR1 levels in qv4J, but not qv3J islets (Fig. 5 E and F). Finally, staining of pancreatic islets revealed similar results, namely, a significant decrease in Kir6.2 pT224 expression in islets from qv3J mice compared with WT mice (Fig. S6). These data support the hypothesis that βIV-spectrin is critical for the regulation of KATP channel activity through direct CaMKII phosphorylation of Kir6.2.
Discussion
The KATP channel is a vital component in the regulation of insulin secretion, with mutations resulting in diabetes mellitus or hyperinsulinemia (18). However, little progress has been made regarding the posttranslational modification of the KATP channel. βIV-spectrin is a multifunctional regulatory protein for KATP channels in the islet. Using mutant βIV-spectrin mouse models, we determined that βIV-spectrin is necessary for the expression and targeting of select structural and regulatory proteins in the islet. For example, our data show that βIV-spectrin is critical for expression of the CaMKIIδ polypeptide in islets. Thus, βIV-spectrin fails to coimmunoprecipitate with CaMKIIδ in qv3J islets (Fig. S3) due to both its inability to associate with CaMKIIδ (Fig. 2 C and D) and the reduced steady-state levels of CaMKIIδ polypeptide in the qv3J lysate (Fig. 3). Additionally, we identified a regulatory mechanism for KATP channels: direct phosphorylation of Kir6.2 T224 by CaMKII. Specifically, Kir6.2 T224 phosphorylation results in a significant decrease in IKATP. Finally, using an antibody that specifically recognizes the phosphorylated form of Kir6.2 T224, we demonstrated that qv3J and qv4J islets lack phosphorylated Kir6.2 T224, as well as CaMKIIδ. Collectively, our data define CaMKII as a membrane skeleton component in the pancreatic islet and βIV-spectrin/AnkB as a necessary molecular platform for KATP channel regulation. As discussed below, future experiments to evaluate Kir6.2 T224 phosphorylation status in human and animal models of diabetes/metabolic disease will be important to assess the potential of this target site as a biomarker in the early progression of disease. Further, whereas our data demonstrate that βIV-spectrin associates with CaMKII, ankyrin-B, actin, Kir6.2, and SUR1 in islets, these results do not necessarily support that these proteins reside in a single macromolecular complex. Future experiments to validate a single macromolecular complex would include expanded coimmunoprecipitation studies that (i) verify that antibodies against each of the proteins in the putative complex recovers each of the other components and (ii) demonstrate that recovery of one of the components of the putative complex is prevented in the absence of one of the other molecules.
Whereas this study addresses the specific role of Kir6.2/SUR1 in the islet, KATP channels are widely distributed in the central and peripheral nervous system, pituitary, cardiac muscle, smooth muscle, and skeletal muscle (18, 19) where they regulate such diverse physiological functions as neuronal excitability, hormone secretion, vascular tone, heart rate, and protection of cells against metabolic stress (18). Notably, human KCNJ11 gene variants result in developmental delay, epilepsy, and neonatal diabetes (DEND) due to overactive KATP channels in muscle, peripheral nerves, and brain.
In heart, KATP channels serve a protective role by activating under metabolic stress and leading to a shortening of the action potential. To this end, KATP channels serve as protectors against cardiac maladaptation to stress, as indicated by hearts deficient in KATP channels being more susceptible to Ca2+-dependent maladaptive remodeling, progressing to organ failure and death. Whereas KCNJ11 polymorphisms have been associated with cardiac disease, no clear gene mutations have been directly linked with severe forms of arrhythmia or myopathy to date.
In addition to regulation by intracellular nucleotides, KATP channel activity, and consequently its physiological function, can be modified by protein phosphorylation. For example, CaMKII activity has been linked with KATP channel regulation in heart and brain (17, 20). KATP channels are also modulated by cAMP-dependent protein kinase (PKA) (21), Ca2+/phospholipid-dependent protein kinase (PKC) (22), extracellular signal-regulated kinase (ERK) (23), and cGMP-dependent protein kinase (PKG) (24). Prior work from Jan and coworkers identified Kir6.2 T224 as a site for PKA-dependent phosphorylation in HEK293 cells (25). Likely, both CaMKII and PKA will have functional roles on Kir6.2 regulation in vivo, although our data demonstrate that CaMKII inhibition is sufficient to ameliorate Kir6.2 pT224 phosphorylation in isoproterenol-treated INS1 cells.
Whereas our findings identify βIV-spectrin as a component in the regulation of KATP channel activity in the islet, they also raise many questions regarding the specific role of CaMKII-mediated phosphorylation. There is a growing body of evidence from enzyme activation studies in support of a role for CaMKII in insulin secretion. Specifically, there is a correlation between CaMKII activation with glucose-stimulated insulin secretion in rat islets (26). CaMKII activation in the beta cell has been linked to phosphorylation of proteins that are involved in secretory events, namely, microtubule-associated protein-2 and synapsin I. Whereas the phosphorylation of proteins involved in granule exocytosis has been identified, little work has been performed to define a role for CaMKII in the regulation of beta cell ion channel function. Previous studies have demonstrated that suppression of cardiac CaMKII, via the transgenic expression of a CaMKII inhibitor, results in an augmentation of KATP-dependent current (17). We have found that expression of an active form of CaMKII produces a significant decrease in IKATP in a heterologous expression system, suggesting that CaMKII-mediated phosphorylation of the KATP channel results in a decrease in IKATP, whereas inhibition of CaMKII results in an increase in IKATP. Two interesting questions for future evaluation are raised based on this finding. First, what is the physiological consequence of CaMKII-mediated phosphorylation of pancreatic KATP channels? CaMKII-dependent regulation of KATP channels may play multiple roles in KATP channel targeting, KATP channel plasma membrane density, and KATP channel biophysical regulation. Studies in heart have demonstrated that suppression of CaMKII activity results in an augmentation of IKATP due to a change in sarcolemmal KATP channel density (17). Future studies to discern specific consequences of CaMKII-mediated KATP channel phosphorylation (i.e., single channel kinetics, ATP sensitivity, and/or plasma membrane density/stability) will be important to provide insight into the specific roles of CaMKII in beta cell KATP channel modulation and potential regulation of insulin secretion. As noted above, CaMKII-mediated phosphorylation of the KATP channel results in a decrease in IKATP, therefore we hypothesize CaMKII activation to decrease glucose-stimulated insulin secretion. Considering that previous studies have shown a direct relationship between the activation of pancreatic CaMKII and glucose-stimulated insulin secretion, it will be important to eventually evaluate glucose-stimulated insulin secretion in qv3J and qv4J mice that have disrupted βIV-spectrin–mediated CaMKII expression and localization. However, for these studies, it will be important to consider the inherent physiological differences for insulin regulation in mice vs. humans (i.e., Kir6.2 null mice do not display hypoglycemia, but display increased insulin sensitivity and low insulin levels). It will be interesting to evaluate the effect of CaMKII inhibition on KATP channel ATP sensitivity, which represents a critical factor in KATP channel regulation. Second, what is the localization of KATP channel populations targeted by CaMKII? Whereas we and others have observed Kir6.2 and SUR1 across the beta cell (immunostaining throughout cytosol and membrane, Figs. 1 and 3), KATP channel subunits have also been observed at the insulin granule (27). Compared with the HEK cell or cardiomyocyte, the pancreatic beta cell is small with few well-defined membrane features. Therefore, future experiments using high-resolution double immunogold labeling of the beta cell for CaMKII and KATP channel subunits will be necessary to resolve this issue. In fact, beyond CaMKII and KATP channel subunits, our data demonstrate multiple heterogeneous subpopulations of spectrin and ankyrin in beta cells (with or without colocalization with insulin). It will be interesting for future studies to define the specific localization, molecular identities, and functions of these subpopulations.
In summary, our findings demonstrate a role for βIV-spectrin in the targeting and expression of CaMKII in the pancreatic islet. Further, the direct coupling of βIV-spectrin to AnkB potentially localizes KATP channel subunits in immediate proximity with CaMKII for direct Kir6.2 phosphorylation (Kir6.2 T224). We predict that these proteins play key roles in vertebrate excitable cell physiology, although this regulation is likely complex. For example, we previously demonstrated that a human Kir6.2 E322K permanent neonatal diabetes mutation that blocks AnkB association is associated with both defects in membrane trafficking and Kir6.2 membrane biophysical activity (12). However, Tarasov et al. found that this site may also be involved in coupling Kir6.2 regulation with SUR1 (15), and individuals with the KCNJ11 E322K mutation respond to sulfonylurea treatment. Whereas additional data will be necessary to define the mechanisms underlying the regulation of these proteins at baseline and in disease, these findings ultimately support the clear in vivo roles of the cytoskeleton and cytoskeletal-associated proteins in the regulation of excitable cell function.
Materials and Methods
Kir6.2 mutants (S37A, S37E, T224A, T224E, S372A, and S372E) were generated using the Stratagene QuikChange site-directed mutagenesis kit, manufacturer’s protocols, and unique primers.
Additional materials and methods are found in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health Grants HL096805 and HL114893 (to T.J.H.); HL084583 and HL083422 (to P.J.M.); HL079031, HL096652, and HL070250 (to M.E.A.); and the Gilead Scholars Program (T.J.H.), the American Heart Association (P.J.M.), the Saving Tiny Hearts Society (P.J.M.), and the Fondation Leducq (08CVD01) (to M.E.A. and P.J.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314195110/-/DCSupplemental.
References
- 1.Machnicka B, Grochowalska R, Bogusławska DM, Sikorski AF, Lecomte MC. Spectrin-based skeleton as an actor in cell signaling. Cell Mol Life Sci. 2012;69(2):191–201. doi: 10.1007/s00018-011-0804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett V, Healy J. Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harb Perspect Biol. 2009;1(6):a003012. doi: 10.1101/cshperspect.a003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berghs S, et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151(5):985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schafer DP, et al. (2009) Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J Neurosci 29(42):13242–13254. [DOI] [PMC free article] [PubMed]
- 5.Lacas-Gervais S, et al. BetaIVSigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. J Cell Biol. 2004;166(7):983–990. doi: 10.1083/jcb.200408007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J Neurosci. 2004;24(33):7230–7240. doi: 10.1523/JNEUROSCI.2125-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkinson NJ, et al. Mutant beta-spectrin 4 causes auditory and motor neuropathies in quivering mice. Nat Genet. 2001;29(1):61–65. doi: 10.1038/ng710. [DOI] [PubMed] [Google Scholar]
- 8.Yang E, Schulman H. Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J Biol Chem. 1999;274(37):26199–26208. doi: 10.1074/jbc.274.37.26199. [DOI] [PubMed] [Google Scholar]
- 9.Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275(31):23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 10.Hund TJ, et al. A β(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120(10):3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy SP, Warren SL, Forget BG, Morrow JS. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol. 1991;115(1):267–277. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kline CF, et al. Dual role of K ATP channel C-terminal motif in membrane targeting and metabolic regulation. Proc Natl Acad Sci USA. 2009;106(39):16669–16674. doi: 10.1073/pnas.0907138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healy JA, et al. Cholinergic augmentation of insulin release requires ankyrin-B. Sci Signal. 2010;3(113):ra19. doi: 10.1126/scisignal.2000771. [DOI] [PubMed] [Google Scholar]
- 14. Kline CF, Hund TJ, Mohler PJ (2010) Ankyrin regulates KATP channel membrane trafficking and gating in excitable cells. Channels (Austin) 4(1):55–57. [DOI] [PMC free article] [PubMed]
- 15.Tarasov AI, et al. Functional analysis of two Kir6.2 (KCNJ11) mutations, K170T and E322K, causing neonatal diabetes. Diabetes Obes Metab. 2007;9(Suppl 2):46–55. doi: 10.1111/j.1463-1326.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Kline CF, Hund TJ, Anderson ME, Mohler PJ. Ankyrin-B regulates Kir6.2 membrane expression and function in heart. J Biol Chem. 2010;285(37):28723–28730. doi: 10.1074/jbc.M110.147868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, et al. (2007) Calmodulin kinase II inhibition enhances ischemic preconditioning by augmenting ATP-sensitive K+ current. Channels (Austin) 1(5):387–394. [DOI] [PubMed]
- 18.Ashcroft FM. The Walter B. Cannon Physiology in Perspective Lecture, 2007. ATP-sensitive K+ channels and disease: From molecule to malady. Am J Physiol Endocrinol Metab. 2007;293(4):E880–E889. doi: 10.1152/ajpendo.00348.2007. [DOI] [PubMed] [Google Scholar]
- 19.Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- 20.Yan XS, Ma JH, Zhang PH. Modulation of K(ATP) currents in rat ventricular myocytes by hypoxia and a redox reaction. Acta Pharmacol Sin. 2009;30(10):1399–1414. doi: 10.1038/aps.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507(Pt 1):117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Light PE, Allen BG, Walsh MP, French RJ. Regulation of adenosine triphosphate-sensitive potassium channels from rabbit ventricular myocytes by protein kinase C and type 2A protein phosphatase. Biochemistry. 1995;34(21):7252–7257. doi: 10.1021/bi00021a041. [DOI] [PubMed] [Google Scholar]
- 23.Lin YF, Chai Y. Functional modulation of the ATP-sensitive potassium channel by extracellular signal-regulated kinase-mediated phosphorylation. Neuroscience. 2008;152(2):371–380. doi: 10.1016/j.neuroscience.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Ropero AB, et al. Non-genomic actions of 17beta-oestradiol in mouse pancreatic beta-cells are mediated by a cGMP-dependent protein kinase. J Physiol. 1999;521(Pt 2):397–407. doi: 10.1111/j.1469-7793.1999.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J. 2000;19(5):942–955. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenham RM, Landt M, Easom RA. Glucose activates the multifunctional Ca2+/calmodulin-dependent protein kinase II in isolated rat pancreatic islets. J Biol Chem. 1994;269(7):4947–4952. [PubMed] [Google Scholar]
- 27.Geng X, Li L, Watkins S, Robbins PD, Drain P. The insulin secretory granule is the major site of K(ATP) channels of the endocrine pancreas. Diabetes. 2003;52(3):767–776. doi: 10.2337/diabetes.52.3.767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.