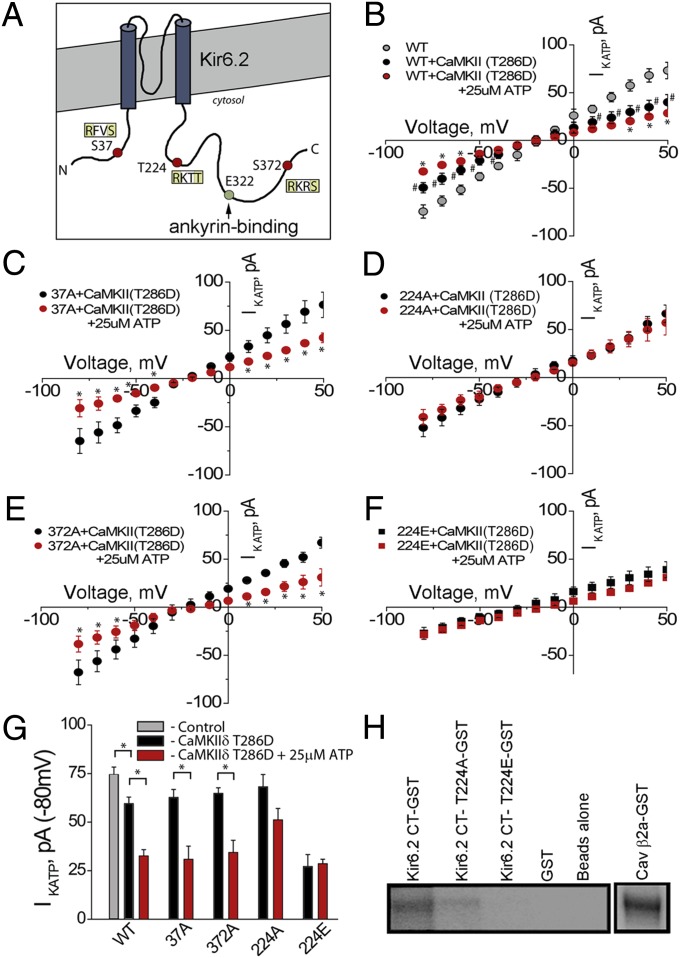
Fig. 4.
CaMKII regulates KATP channel function. (A) Kir6.2 with AnkB-binding site denoted at Kir6.2 E322. Image denotes three putative CaMKII-phosphorylation sites: S37, T224, and S372. (B) COSm6 cells transfected with Kir6.2/CaMKII T286D demonstrate a significant reduction in IKATP, compared with control, ±ATP (25 μM). Likewise, we observed significant reductions in IKATP in cells transfected with CaMKII T286D and Kir6.2 S37A (C) and Kir6.2 S372A (E), ±25 μM ATP. We observed no significant change in IKATP in cells transfected with CaMKII T286D/Kir6.2 T224A, ±25 μM ATP (D). (F) Finally, transfection with CaMKII T286D/Kir6.2 T224E (phosphomimetic) demonstrates a significant reduction in IKATP compared with control, but no significant difference with and without ATP administration. (G) Summary of functional IKATP data (n > 7 cells per treatment; P < 0.05). (H) Purified and immobilized Kir6.2, Kir6.2 T224A, and Kir6.2 T224E were assessed for CaMKII-mediated phosphorylation in vitro. Control (β2a) and Kir6.2 showed robust CaMKII-mediated phosphorylation. There was significantly reduced phosphorylation of Kir6.2 T224A and Kir6.2 T224E.