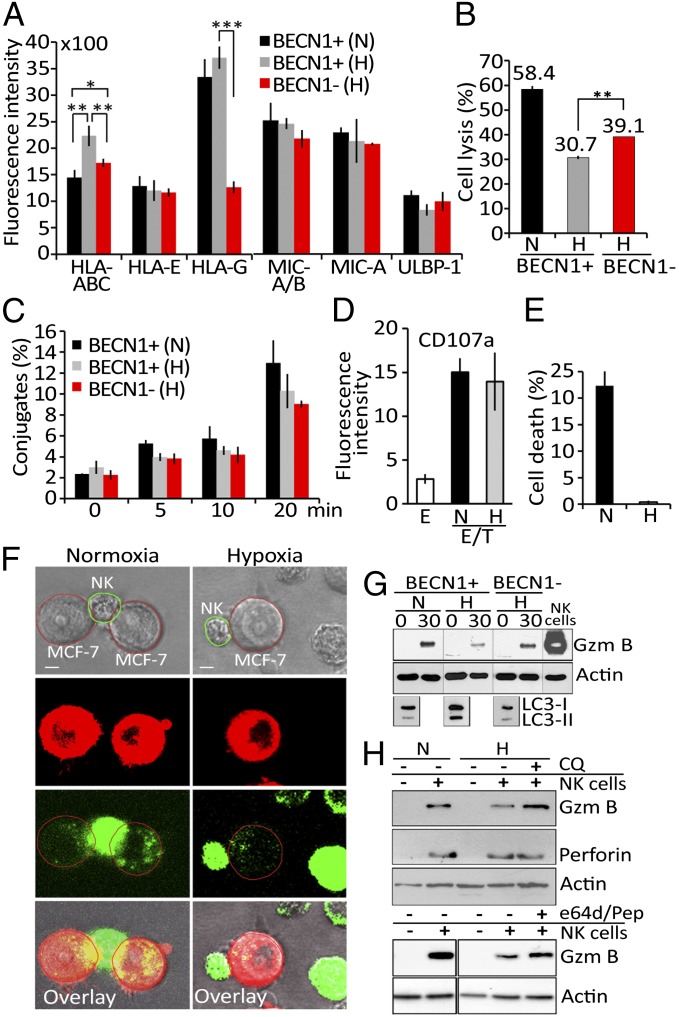
Fig. 2.
Hypoxia-induced autophagy impairs tumor cell susceptibility to NK-mediated lysis without affecting NK cell function. (A) Autophagy-competent (BECN1+) and -defective (BECN1−) MCF-7 cells cultured under normoxia (N) or hypoxia (H) were assessed by flow cytometry for the expression of MHC class I molecules and NK cell-activating NKG2D ligands . Fluorescence intensity is reported as an average (±SEM) of three experiments. Statistically significant differences are indicated by asterisks (*P < 0.05; **P < 0.005; ***P < 0.0005). (B) BECN1+ or BECN1− MCF-7 cells were pretreated with mouse IgM anti-pan HLA class I A6-136 mAb and incubated under normoxic (N) or hypoxic (H) conditions before presentation to NK cells at a 5/1 E/T ratio. The percentage of target cell lysis is reported. Statistically significant differences are indicated by asterisks (**P < 0.005). (C) BECN1+ or BECN1− MCF-7 cells cultured under normoxia (N) or hypoxia (H) were incubated with NK cells. The percentage of conjugate formation at indicated time was determined by flow cytometry. Results are reported as an average (±SEM) of three independent experiments. No statistically significant differences were observed. (D) NK cells as effectors (E) were cultured alone or with normoxic (N) or hypoxic (H) MCF-7 cells at a 5/1 E/T ratio. The level of CD107a (a degranulation marker) on the surface of the NK cells was assessed by flow cytometry. Fluorescence intensity is reported as an average (±SEM) of five experiments performed with NK cells from different donors. (E) Normoxic (N) or hypoxic (H) MCF-7 cells were loaded with exogenous, activated granzyme B (0.8 μg/mL) using the pore-forming protein streptolysin-O. The percentage of cell death was determined by flow cytometry. Results are reported as an average (±SEM) of three experiments. (F) PKH-26–stained normoxic or hypoxic MCF-7 cells (red) were cocultured with YT–Indy–NK cells expressing GFP–GzmB (green) at 5/1 E/T ratio. The content of NK-derived GFP–GzmB in target cells was monitored after 30 min of coculture by a Zeiss laser-scanning confocal microscope (LSM-510 Meta) with a 60× oil immersion objective. (Scale bar, 10 μm.) (G) Autophagy-competent (BECN1+) or -defective (BECN1−) MCF-7 cells were incubated under normoxia (N) or hypoxia (H) and cocultured with NK cells at 5/1 E/T ratio for 0 and 30 min. Following separation, tumor cells were lysed and subjected to immunoblot for the intracellular GzmB content. NK cell lysate was used as a control for GzmB detection. The expression of LC3 was reported as a marker for autophagy. (H) Normoxic (N) or hypoxic (H) MCF-7 cells were cultured alone (−) or with NK cells (+) at 5/1 ratio for 30 min in the presence (+) or absence (−) of chloroquine (CQ) or e64d/pepstatin. Tumor cells separated from NK were subjected to immunoblot analysis to evaluate the intracellular GzmB and perforin content.