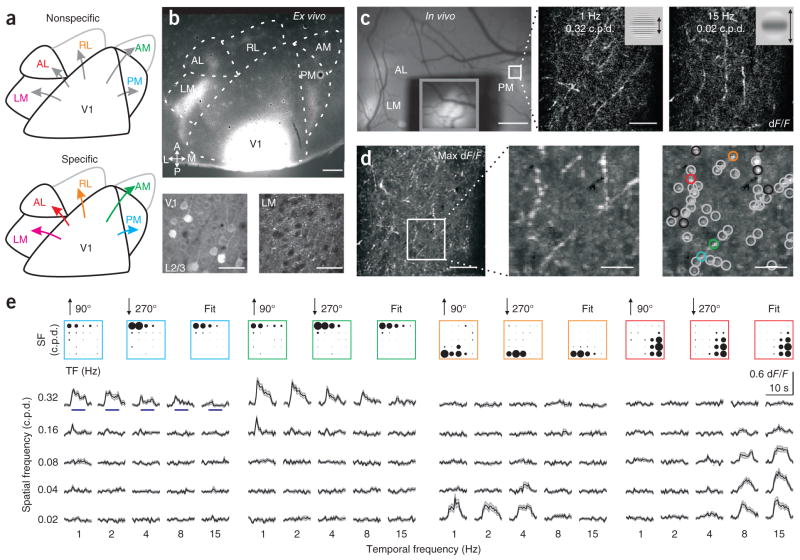
Figure 1.
Functional two-photon calcium imaging from the axons of V1 projection neurons. (a) Two models of mouse visual cortex. Higher visual areas may receive functionally nonspecific (top) or specific (bottom) inputs from V1. Specificity may arise through diverse mechanisms, including a bias in projection probability, arborization size or neural excitability. (b) Labeling of V1 axonal projections with GCaMP3.3. Top, tangential section of visual cortex; A, anterior; P, posterior; L, lateral; M, medial. Bottom, infected somata in layer 2/3 (L2/3) of V1 (left) and V1 axonal arborizations in LM (right). Scale bars, 500 μm (top) and 30 μm (bottom). (c) In vivo calcium imaging. Left, in vivo image of visual cortex. V1 was covered to prevent saturation and the inset (gray box) taken with lower illumination. Middle and right, example average two-photon fluorescence responses (dF/F; 24 trials) of axons in PM (white outlined region in left panel). Stimuli (insets) are sinusoidal drifting gratings of different spatial and temporal frequencies. Scale bars, 500 μm (left) and 50 μm (middle and right). (d) Identification of visually responsive boutons. Left, maximum (max) response projection across stimuli. Middle, magnification of the boxed region at left. Right, gray and colored circles indicate locations of boutons whose tunings are well fit by a two-dimensional Gaussian. Black circles indicate poor fits (excluded from further analysis). Scale bars, 50 μm (left) and 15 μm (middle and right). (e) Visual responses of boutons in colored circles from d. Top, average response of each bouton (for the indicated directions of motion; 12 trials per stimulus) and the fit of the average response (right). The area of each circle is proportional to dF/F. SF, spatial frequency; TF, temporal frequency. Bottom, average dF/F time course for each stimulus. Blue lines (in left panel) represent duration of stimulus (5 s). Shaded regions are ± s.e.m.