Figure 1.
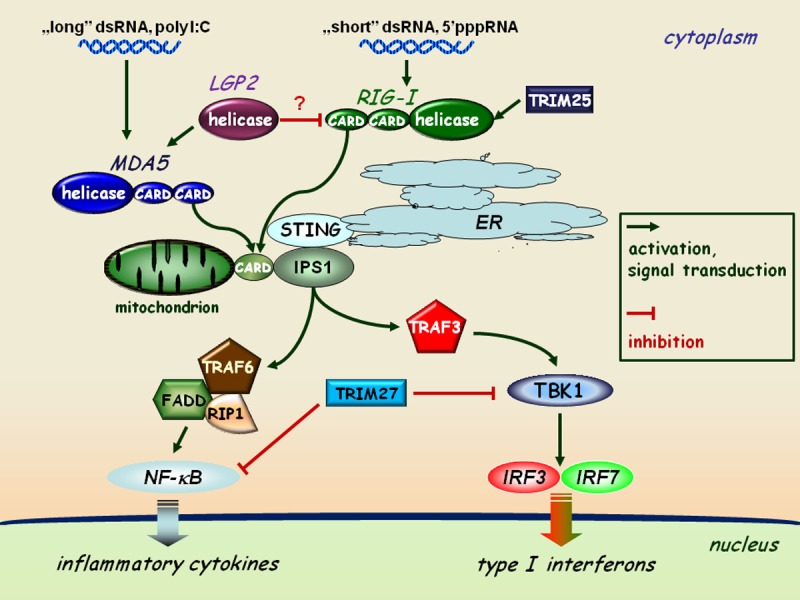
RLR-mediated pathways of type I interferon and inflammatory responses. The interaction of dsRNA as a viral genome or as a replication intermediate of RNA viruses with the helicase domain of RLRs (RIG-I or MDA5) induces association of the CARD domains of RIG-I/MDA5 and the adaptor protein IPS1 localized to the mitochondrial membrane. This receptor-adaptor interaction results in the activation of TBK1 and the subsequent phosphorylation of IRF3 and IRF7 on specific serine residues, resulting in their homodimerization. These dimers can translocate to the cell nucleus and activate the transcription of type I IFN genes. The expression of IRF3, IRF7, RIG-I and MDA5 is coordinately upregulated by type I IFN-mediated signaling acting as an amplification circuit. This pathway together with IPS1 is coupled to the NF-κB signaling pathway through the interaction of FADD (FAS-associated via death domain), RIP1, and TRAF6 resulting in the induction of inflammatory cytokine genes, such as IL-1β, IL-6 and TNFα. The TRIM proteins shown act as specific regulators of this pathway.
