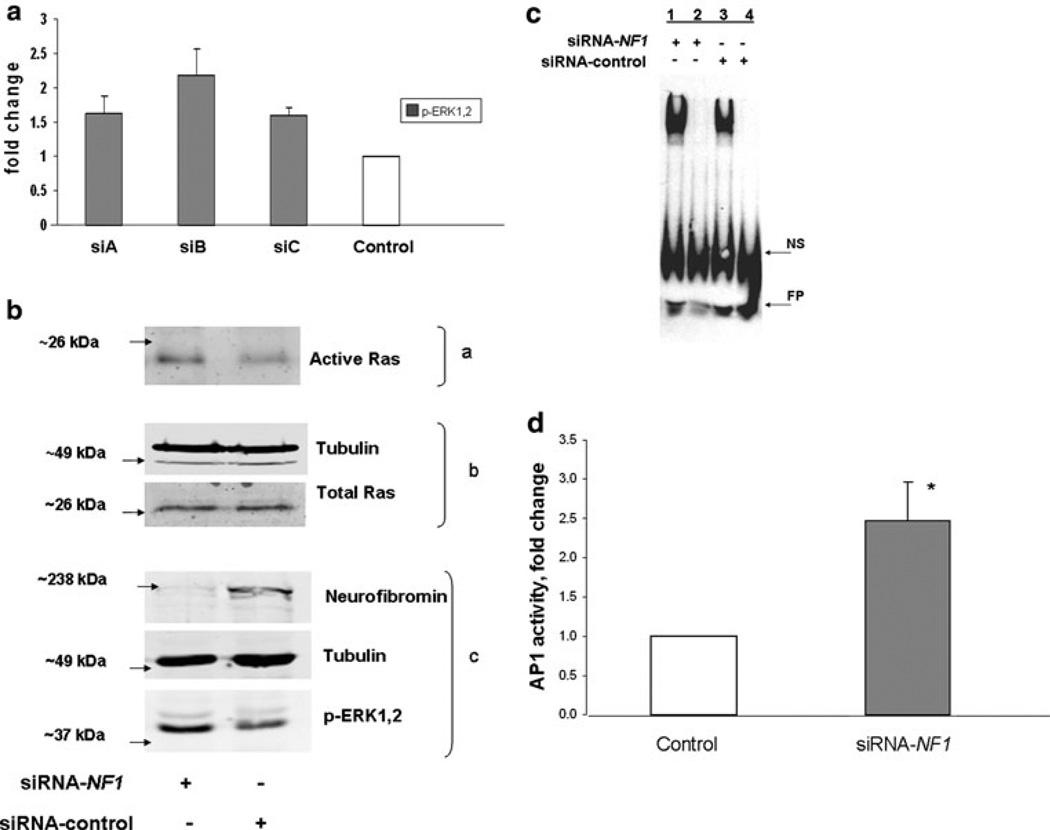
Fig. 1.
Neurofibromin knockdown increases active Ras, active ERK1,2, AP-1 binding and activity. a Three siRNA-NF1 duplexes were tested for effectiveness in upregulating the ERK1,2 pathway in STS26T. Although not significant, upregulation of ERK1,2 activity was highest with siB and this siRNA was used in subsequent experiments. Data are representative of three independent experiments and expressed as means ± SEM. b STS26T cultures were transfected with siRNA-NF1 or siRNA-control followed by harvest for western blot analysis 48 h later. Two-thirds of the cell lysate was subjected to affinity purification for active Ras (a) with the Ras-binding domain of Raf coupled to glutathione S-transferase; one-third of the lysate was reserved for determining total Ras and tubulin (b) and neurofibromin, tubulin and active ERK1,2 (c). Data are representative of three independent experiments. c STS-26T cells were transfected with siRNA, NF1 or control, and harvested 48 or 72 h post-transfection. EMSAs were performed with nuclear extracts of cells transfected with siRNA-NF1 or control siRNA. Competitive binding reactions with excess unlabeled probe were added to NF1 or control samples in lanes 2 and 4, respectively. Data are representative of three independent experiments. NS non-specific binding, FP free probe. d STS-26T cells were transfected with siRNA-NF1 or scrambled control duplexes followed 24 h later by transfection with the AP-1 luciferase reporter plasmids. Cell lysates for dual luciferase assay were prepared 48 h after second transfection. Data are presented as the fold activation relative to the activity obtained with the scrambled siRNA control. Values represent the mean ± SEM for three independent experiments. AP-1 activity in NF1 knockdown cells was found to be significantly different (* P value < 0.05) than control cells