Fig. 2.
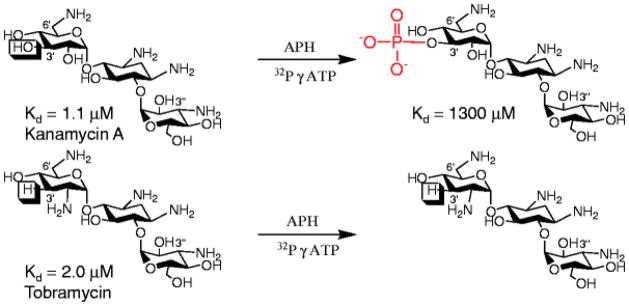
The structures of the aminoglycosides kanamycin A and tobramycin, their measured affinity to a mimic of the bacterial rRNA A-site (26) and the product of their modification by APH(3′)-IIIa. Note that kanamycin A is modified by APH(3′) because it contains a reactive hydroxyl group at the 3′ position, whereas tobramycin contains a hydrogen atom and is thus not susceptible to APH(3′) modification.
